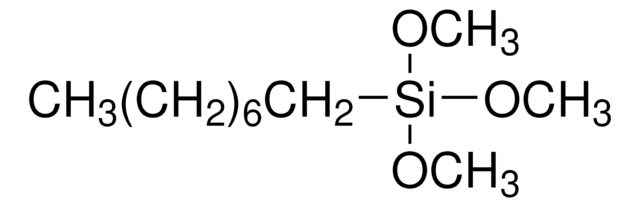All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H9N
CAS Number:
Molecular Weight:
83.13
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
refractive index
n20/D 1.444 (lit.)
bp
104-105 °C (lit.)
density
0.878 g/mL at 25 °C (lit.)
SMILES string
CC1=NCCC1
InChI
1S/C5H9N/c1-5-3-2-4-6-5/h2-4H2,1H3
InChI key
CTSZPNIMMLSKDV-UHFFFAOYSA-N
General description
2-Methyl-1-pyrroline, a monocyclic imine, is a pyrroline derivative. It is a five-membered heterocyclic compound having various biological and pharmacological applications. It is formed during the Rh(I) complexes (containing N,N-donor ligands and N,P-donor ligand) immobilized on glassy carbon electrode surfaces catalyzed intramolecular hydroamination of 4-pentyn-1-amine. It reacts with with 2-oxopropanal to afford acetyl-1-pyrroline (AP).
Application
2-Methyl-1-pyrroline (2-MPN) may be used in the enantioselective enzymatic synthesis of (R)- and (S)-2-methylpyrroline in the presence of whole-cells catalysts isolated from Strptomyces sp. strains GF3587 and GF3546 respectively.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
51.8 °F - closed cup
Flash Point(C)
11 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M Bertoldi et al.
The Biochemical journal, 342 Pt 3, 509-512 (1999-09-08)
Ornithine decarboxylase (ODC) from Lactobacillus 30a catalyses the cleavage of alpha-methylornithine into ammonia and 2-methyl-1-pyrroline; glutamate decarboxylase (GAD) from Escherichia coli catalyses the cleavage of alpha-methylglutamate into ammonia and laevulinic acid. In our analyses, 2-methyl-1-pyrroline and laevulinic acid were identified
María Rodríguez-Mata et al.
Chembiochem : a European journal of chemical biology, 14(11), 1372-1379 (2013-07-03)
NADPH-dependent oxidoreductase Q1EQE0 from Streptomyces kanamyceticus catalyzes the asymmetric reduction of the prochiral monocyclic imine 2-methyl-1-pyrroline to the chiral amine (R)-2-methylpyrrolidine with >99% ee, and is thus of interest as a potential biocatalyst for the production of optically active amines.
Andrey A Tregubov et al.
Journal of the American Chemical Society, 135(44), 16429-16437 (2013-10-04)
A series of N,N-donor ligands (bis(pyrazol-1-yl)methane (bpm), bis(N-methylimidazol-2-yl)methane (bim), 1-(phenylmethyl)-4-(1H-pyrazol-1-yl methyl)-1H-1,2,3-triazole (PyT)), and one N,P-donor ligand precursor (1-(3,5-dimethylpyrazol-1-yl)(2-bromoethane) (dmPyBr)) were synthesized and functionalized with aniline. Diazotization of the aniline into an aryl diazonium, using nitrous acid in aqueous conditions, was
2-Oxopropanal, hydroxy-2-propanone, and 1-pyrroline important intermediates in the generation of the roast-smelling food flavor compounds 2-acetyl-1-pyrroline and 2-acetyltetrahydropyridine.
Hofmann T and Schieberle P.
Journal of Agricultural and Food Chemistry, 46(6), 2270-2277 (1998)
Koichi Mitsukura et al.
Bioscience, biotechnology, and biochemistry, 75(9), 1778-1782 (2011-09-08)
The (R)-imine reductase (RIR) of Streptomyces sp. GF3587 was purified and characterized. It was found to be a NADPH-dependent enzyme, and was found to be a homodimer consisting of 32 kDa subunits. Enzymatic reduction of 10 mM 2-methyl-1-pyrroline (2-MPN) resulted
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service