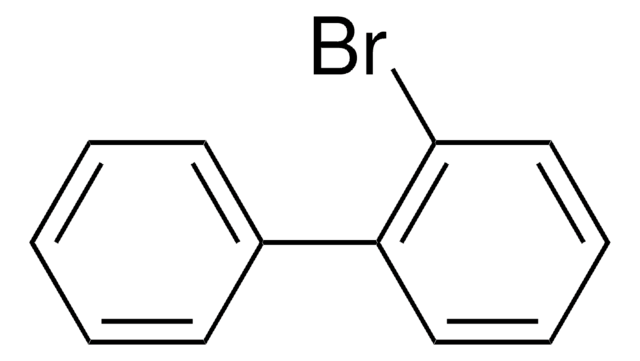
All Photos(1)
About This Item
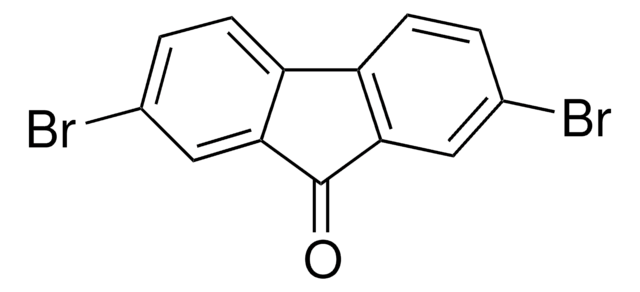
Empirical Formula (Hill Notation):
C13H7BrO
CAS Number:
Molecular Weight:
259.10
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
form
solid
mp
146-148 °C (lit.)
SMILES string
Brc1ccc2-c3ccccc3C(=O)c2c1
InChI
1S/C13H7BrO/c14-8-5-6-10-9-3-1-2-4-11(9)13(15)12(10)7-8/h1-7H
InChI key
MTCARZDHUIEYMB-UHFFFAOYSA-N
Application
2-Bromo-9-fluorenone was used:
- as end-capping agent for poly(9,9-dialkylfluorene-2,7-diyl) derivatives
- in preparation of spirobifluorene ligands
- in synthesis of altitudinal molecular motors which contain functional groups in their rotor part
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Oxidative stability and its effect on the photoluminescence of poly (fluorene) derivatives: end group effects.
Lee J-I, et al.
Chemistry of Materials, 11(4), 1083-1088 (1999)
Novel iridium complexes as high-efficiency yellow and red phosphorescent light emitters for organic light-emitting diodes.
Yao JH, et al.
Tetrahedron, 64(48), 10814-10820 (2008)
Gábor London et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 19(32), 10690-10697 (2013-06-21)
We report the synthesis of altitudinal molecular motors that contain functional groups in their rotor part. In an approach to achieve dynamic control over the properties of solid surfaces, a hydrophobic perfluorobutyl chain and a relatively hydrophilic cyano group were
Guang-Wei Zhang et al.
International journal of molecular sciences, 14(11), 22368-22379 (2013-11-16)
Supramolecular luminescence stems from non-covalent exciton behaviors of active π-segments in supramolecular entities or aggregates via intermolecular forces. Herein, a π-conjugated oligofluorenol, containing self-complementary double hydrogen bonds, was synthesized using Suzuki coupling as a supramolecular semiconductor. Terfluorenol-based random supramolecular polymers
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service