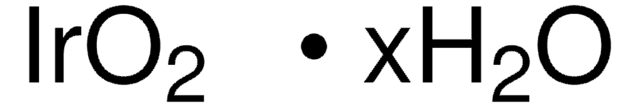All Photos(1)
About This Item
Empirical Formula (Hill Notation):
Ir
CAS Number:
Molecular Weight:
192.22
EC Number:
MDL number:
UNSPSC Code:
12141720
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Assay
99.9% trace metals basis
form
powder
resistivity
4.71 μΩ-cm
bp
4130 °C (lit.)
mp
2450 °C (lit.)
density
22.65 g/cm3 (lit.)
SMILES string
[Ir]
InChI
1S/Ir
InChI key
GKOZUEZYRPOHIO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Iridium is a rare and dense transition metal that belongs to the platinum group of metals and is known for its remarkable physical and chemical properties. Iridium is used in high-temperature applications such as crucibles for crystal growth and in devices that operate under extreme conditions due to its high melting point and stability at elevated temperatures. It is also used in electrical contacts and electrodes because of its excellent corrosion resistance and electrical conductivity. In addition, iridium is used as a catalyst in various organic reactions including hydrogenation, water splitting, C-C coupling, and olefin metathesis.
Application
- Iridium-based double perovskites for efficient water oxidation in acid media: This study highlights the development of iridium-based double perovskites that reduce iridium content while maintaining high activity and stability for water oxidation, significant for energy-related applications in material science (Diaz-Morales et al., 2016).
- Recent advances in understanding oxygen evolution reaction mechanisms over iridium oxide: This paper provides insights into the oxygen evolution reaction (OER) mechanisms on iridium oxide, crucial for improving catalytic processes in industrial applications (Naito et al., 2021).
- Iridium oxide fabrication and application: A review: A comprehensive review that discusses various methods for the fabrication of iridium oxides and their applications, particularly in sensors and catalysts, relevant to both drug discovery and material science (Chen et al., 2020).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Sol. 1
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
nwg
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Rui Cao et al.
Journal of medicinal chemistry, 56(9), 3636-3644 (2013-04-19)
The cellular behavior and toxicity effect of organometallic complexes depend largely on their peripheral ligands. In this study, we have synthesized a series of novel luminescent cationic iridium(III) complexes by tuning the ancillary N(∧)N ligand based on a structure [Ir(ppy)2(N(∧)N)](+)
Shiguang Pan et al.
Organic letters, 15(8), 1902-1905 (2013-04-02)
A cationic Ir(I) complex-catalyzed O-to-N-alkyl migration in 2-alkoxypyridines bearing a secondary alkyl group on the oxygen atom by C-O bond cleavage is described. The present transformation gave various N-alkylpyridones in moderate to good yields. The addition of sodium acetate played
M Y Zhang et al.
Optics express, 21 Suppl 1, A173-A178 (2013-02-15)
We have investigated the transport characteristics of red phosphorescent dye bis(1-(phenyl)isoquinoline) iridium (III) acetylanetonate (Ir(piq)₂acac) doped 4,4',4"-tri(N-carbazolyl)triphenylamine (TCTA), and found that the increasing doping ratio was facilitated to improve the ability of hole transporting. A high color rendering index (CRI)
Matthew R Kelley et al.
Inorganic chemistry, 52(5), 2564-2580 (2013-02-21)
A series of seven [Ir{ArNC(NR2)NAr}(cod)] complexes (1a-1g; where R = Me or Et; Ar = Ph, 4-MeC6H4, 4-MeOC6H4, 2,6-Me2C6H3, or 2,6-(i)Pr2C6H3; and cod = 1,5-cyclooctadiene) were synthesized by two different methods from the neutral guanidines, ArN═C(NR2)NHAr, using either MeLi and
Le Guo et al.
Organic letters, 15(5), 1144-1147 (2013-02-16)
The first α-alkylation of unactivated amides with primary alcohols is described. An effective and robust iridium pincer complex has been developed for selective α-alkylation of tertiary and secondary acetamides involving a "borrowing hydrogen" methodology. The method is compatible with alcohols
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service