182354
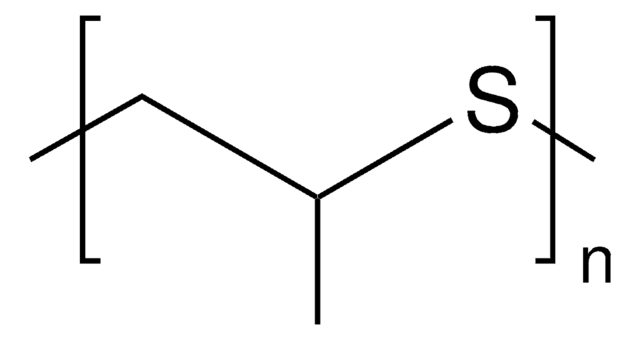
Poly(1,4-phenylene sulfide) average Mn ~ 10,000, powder | 25212-74-2
average Mn ~10,000, powder
Synonym(s):
Poly(1,4-Phenylene Sulfide)
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Recommended Products
form
powder
mol wt
average Mn ~10,000
mp
285-300 °C
transition temp
Tg 150 °C
density
1.36 g/mL at 25 °C
Related Categories
General description
Poly(1,4-phenylene sulfide)(PPS) is an aromatic polymer that can be used as a polymer backbone or an engineering plastic. It is also called as poly(phenylene sulfide) which can be sulfonated to form ion-exchange membranes for electrochemical applications. It is industrially produced by polycondensation of p-dichlorobenzene and sodium sulfate in N-methylpyrrolidone as a solvent.
Application
PPS can be sulfonated to form a proton exchange membrane with zeolite that can be used as an electrolyte with higher thermal stability which facilitates in the fabrication of solar cells. It can also be used in the formation of organic- inorganic nanocomposite membranes using nanozeolites and carbon nanotubes as nanofillers which can be used as high performance gas separation membranes. PPS may be sulfonated and chloromethylated to form novel PPS fiber with higher dyeing ability in dispersive, anionic and cationic dyes.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Dyeing behavior of chemically modified poly (1, 4-phenylene sulfide) fiber towards disperse, anionic, and cationic dyes.
Pervin S, et al.
Fibers and Polymers, 15(6), 1168-1174 (2014)
Crystallization kinetics of polyphenylene sulfide
Jog, J. P., & Nadkarni, V. M.
Journal of Applied Polymer Science, 30(3), 997-1009 (1985)
An optical study of the arsenic pentafluoride doping of poly (p-phenylene sulphide): polaron and bipolaron transitions
Friend, R. H., & Giles, J. R.
Journal of the Chemical Society. Chemical Communications, 16, 1101-1103 (1984)
Design of poly (1-hexadecene-sulfone)/poly (1, 4-phenylene sulfide) membrane containing nano-zeolite and carbon nanotube for gas separation.
Kausar A.
International Journal of Plastics Technology, 21(1), 96-107 (2017)
Crystallization, morphology, and thermal behavior of poly (p-phenylene sulfide).
Silvestre, C., Di Pace, E., Napolitano, R., Pirozzi, B., & Cesario, G.
Journal of Polymer Science. Part B, Polymer Physics, 39(4), 415-424 (2001)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service