All Photos(1)
About This Item
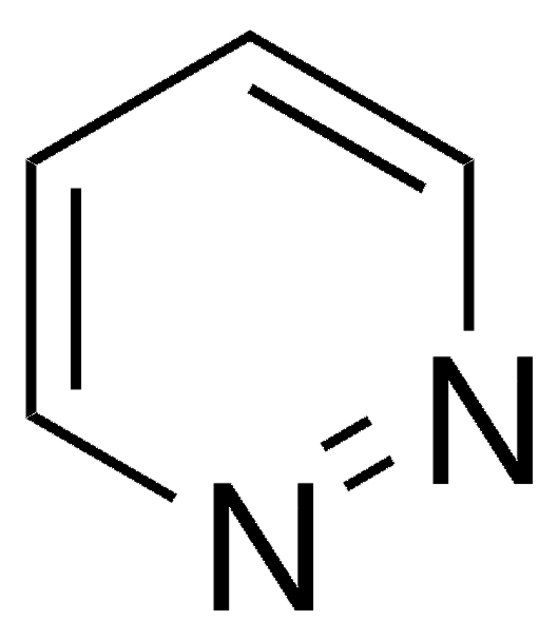
Empirical Formula (Hill Notation):
C5H6N2
CAS Number:
Molecular Weight:
94.11
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
refractive index
n20/D 1.514 (lit.)
bp
214 °C (lit.)
density
1.031 g/mL at 25 °C (lit.)
SMILES string
Cc1cccnn1
InChI
1S/C5H6N2/c1-5-3-2-4-6-7-5/h2-4H,1H3
InChI key
MXDRPNGTQDRKQM-UHFFFAOYSA-N
General description
3-Methylpyridazine(3-Mepydz) reacts with halogenotrimethylplatinum (IV), [PtXMe3)4], to form complexes of type fac-[PtXMe3(3-Mepydz)2] (X = Cl, Br or I). It undergoes self-association in aqueous solution at acidic, neutral and basic pH . It is a diazaaromatic compound and reacts with bis (1,1,1,5,5,5-hexafluoropentane-2,4-dionato) copper (II) complexes.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
190.4 °F - closed cup
Flash Point(C)
88 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A dynamic NMR study of 1, 2-metallotropic shifts in trimethylplatinum (IV) halide complexes fo 3-methylpyridazine.
Abel EW, et al.
Polyhedron, 13(20), 2907-2913 (1994)
H H Borchert et al.
Die Pharmazie, 44(9), 625-630 (1989-09-01)
Pyridazin (1) and 3-methylpyridazine (6) undergo oxidative biotransformation in an unexpected high degree. Beside the unchanged compounds, after administration of 1 two isomeric monohydroxylated products (2, 3), 4,5-dihydrodihydroxypyridazine (4) and 4,5-dihydroxypyridazine (5) and after administration of 6 one ringhydroxylated 6-derivative
Preparation and characterisation of bis (1, 1, 1, 5, 5, 5-hexafluoropentane-2, 4-dionato) copper (II) complexes with diazaaromatic compounds. Part 1. Crystal structures and characterisation of several adducts with diazines.
Kogane T, et al.
J. Chem. Soc., Dalton Trans., 1, 13-18 (1994)
Fernando Peral et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 59(6), 1223-1237 (2003-03-28)
The self-association of pyridazine and pyrazine was studied in aqueous solution at acidic, neutral and basic pH values, by ultraviolet spectroscopy. The spectra of pyridazine in the mid-ultraviolet region did not show any variation in molar absorptivity upon concentration of
Jonita Stankevičiūtė et al.
Scientific reports, 6, 39129-39129 (2016-12-17)
Pyridinols and pyridinamines are important intermediates with many applications in chemical industry. The pyridine derivatives are in great demand as synthons for pharmaceutical products. Moreover, pyridines are used either as biologically active substances or as building blocks for polymers with
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service