810601C
Avanti
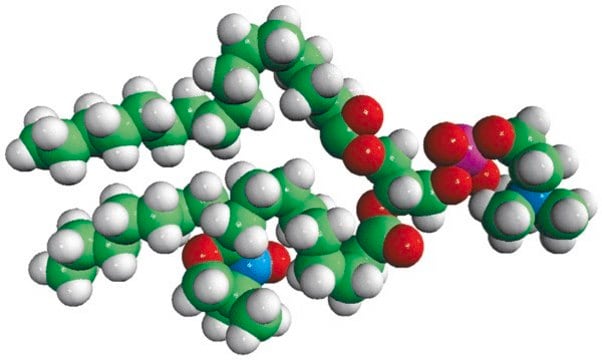
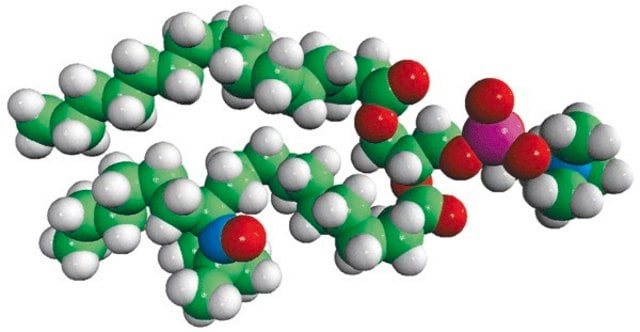
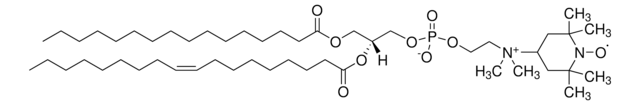
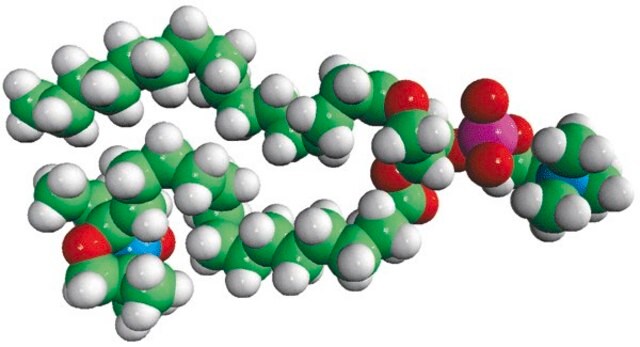
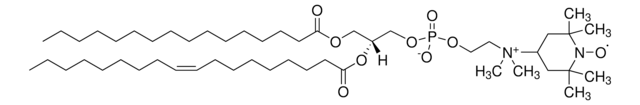
16:0-5 Doxyl PC
Avanti Research™ - A Croda Brand 810601C
Synonym(s):
1-palmitoyl-2-stearoyl-(5-doxyl)-sn-glycero-3-phosphocholine
About This Item
Recommended Products
Assay
>99% (TLC)
form
liquid
packaging
pkg of 1 × 1 mL (810601C-1mg)
manufacturer/tradename
Avanti Research™ - A Croda Brand 810601C
concentration
1 mg/mL (810601C-1mg)
lipid type
ESR probes
phospholipids
shipped in
dry ice
storage temp.
−20°C
General description
Application
- as a component in virus-like large unilamellar vesicles (VL LUVs) to quench 4-chloro-7-nitrobenz-2-oxa-1,3-diazole (NBD) fluorescence emission
- in the preparation of multi-lamellar vesicles (MLVs) as a site-specific quencher to perform fluorescence quenching studies
- in the preparation of spin-labelled multi-lamellar vesicles (MLVs)
Biochem/physiol Actions
Packaging
Preparation Note
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3 - Carc. 2 - Eye Irrit. 2 - Repr. 2 - Skin Irrit. 2 - STOT RE 1 - STOT SE 3
Target Organs
Central nervous system, Liver,Kidney
WGK
WGK 3
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service