I0631
Imidodiphosphate sodium salt
≥97%
Synonym(s):
Tetrasodium imidodiphosphate
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
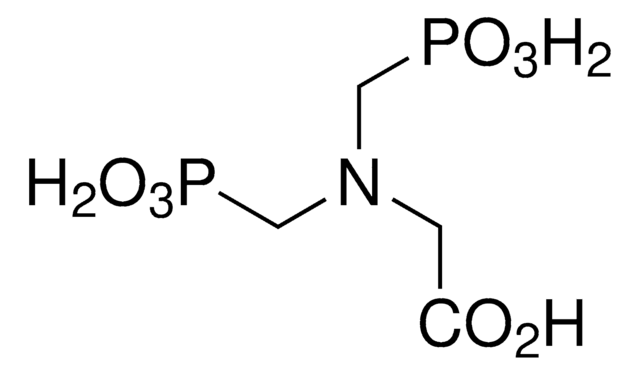
Linear Formula:
HNO6P2Na4
CAS Number:
Molecular Weight:
264.92
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥97%
storage temp.
2-8°C
SMILES string
O=P([O-])([O-])NP([O-])([O-])=O.[Na+].[Na+].[Na+].[Na+]
InChI
1S/H5NO6P2.4Na/c2-8(3,4)1-9(5,6)7;;;;/h(H5,1,2,3,4,5,6,7);;;;/q;4*+1/p-4
InChI key
KDZOOFFPQVQSQG-UHFFFAOYSA-J
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A B Zyryanov et al.
Biochemistry. Biokhimiia, 70(8), 908-912 (2005-10-11)
Imidodiphosphate (the pyrophosphate analog containing a nitrogen atom in the bridge position instead of oxygen) is a potent inhibitor of family II pyrophosphatases from Streptococcus mutans and Streptococcus gordonii (inhibition constant Ki approximately 10 microM), which is slowly hydrolyzed by
Anna M Rydzik et al.
Bioorganic & medicinal chemistry, 20(5), 1699-1710 (2012-02-10)
We describe synthesis and properties of eight dinucleotide mRNA 5' cap analogs containing imidodiphosphate moiety within 5',5'-tri- or tetraphosphate bridge (NH-analogs). The compounds were obtained by coupling an appropriate nucleoside 5'-imidodiphosphate with nucleotide P-imidazolide mediated by divalent metal chloride in
T Rozovskaya et al.
FEBS letters, 247(2), 289-292 (1989-04-24)
It is demonstrated here that rat liver DNA polymerase beta catalyzes the pyrophosphorolysis reaction with pyrophosphate (PPi) and its analogues. The substrate specificity of the PPi-binding site of several DNA polymerases was investigated. It was discovered that the ability of
Highly efficient green and blue-green phosphorescent OLEDs based on iridium complexes with the tetraphenylimidodiphosphinate ligand.
Yu-Cheng Zhu et al.
Advanced materials (Deerfield Beach, Fla.), 23(35), 4041-4046 (2011-07-30)
George D Markham et al.
Biochemistry, 43(12), 3415-3425 (2004-03-24)
S-Adenosylmethionine synthetase (ATP: L-methionine S-adenosyltransferase) catalyzes a two-step reaction in which tripolyphosphate (PPPi) is a tightly bound intermediate. Diimidotriphosphate (O(3)P-NH-PO(2)-NH-PO(3); PNPNP), a non-hydrolyzable analogue of PPPi, is the most potent known inhibitor of AdoMet synthetase with a K(i) of 2
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service