904473
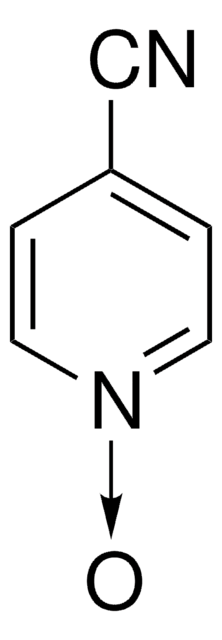
4-Cyano-N-(trifluoromethoxy)pyridinium triflimide
≥95%
Synonym(s):
4-Cyano-1-(trifluoromethoxy)pyridin-1-ium bis((trifluoromethyl)sulfonyl)amide, Togni trifluoromethoxylation reagent
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H4F9N3O5S2
CAS Number:
Molecular Weight:
469.26
UNSPSC Code:
12352101
Recommended Products
Assay
≥95%
form
powder
storage temp.
2-8°C
Application
4-Cyano-N-(trifluoromethoxy)pyridinium triflimide is a bench-stable trifluoromethoxylation reagent developed in the Togni lab. Under irradiation with visible-light in the presence of a photocatalyst, N-O bond cleavage results in an OCF3 radical capable of arene C-H functionalization, providing trifluoromethoxy ethers.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Benson J Jelier et al.
Angewandte Chemie (International ed. in English), 57(42), 13784-13789 (2018-06-22)
A simple trifluoromethoxylation method enables non-directed functionalization of C-H bonds on a range of substrates, providing access to aryl trifluoromethyl ethers. This light-driven process is distinctly different from conventional procedures and occurs through an OCF3 radical mechanism mediated by a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



![1,4-Diazabicyclo[2.2.2]octane ReagentPlus®, ≥99%](/deepweb/assets/sigmaaldrich/product/structures/366/129/a6ff4175-974d-4fac-9038-b35e508ef252/640/a6ff4175-974d-4fac-9038-b35e508ef252.png)



