All Photos(1)
About This Item
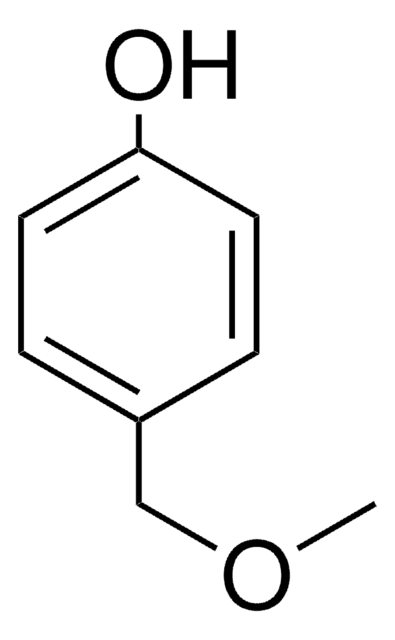
Linear Formula:
CH3O(CH2)2C6H4OH
CAS Number:
Molecular Weight:
152.19
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
42-45 °C (lit.)
SMILES string
COCCc1ccc(O)cc1
InChI
1S/C9H12O2/c1-11-7-6-8-2-4-9(10)5-3-8/h2-5,10H,6-7H2,1H3
InChI key
FAYGEALAEQKPDI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-(2-Methoxyethyl)phenol can be prepared by reacting methyl vinyl ether and 4-bromonitrobenzene.
Application
4-(2-Methoxyethyl)phenol may be used in the preparation of methyl analog of metoprolol (MAM).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A New Route to 4-(2-Methoxyethyl) Phenol Via Palladium-Catalysed Arylation of Methyl Vinyl Ether.
Hallberg A, et al.
Synthetic Communications, 15(13), 1131-1136 (1985)
M Allaoua et al.
Journal of applied microbiology, 125(4), 1162-1174 (2018-05-18)
In vitro and in vivo studies were conducted to test a new carvacrol-based product designed to delay the carvacrol release so that it could reach the caeca of broiler chickens in order to control Campylobacter jejuni. Antimicrobial activity of carvacrol, a
Evidence that serine 304 is not a key ligand-binding residue in the active site of cytochrome P450 2D6.
Ellis SW, et al.
The Biochemical Journal, 345(3), 565-571 (2000)
Alfred Svan et al.
Journal of mass spectrometry : JMS, 51(3), 207-218 (2016-03-10)
Identification of degradation products from trace organic compounds, which may retain the biological activity of the parent compound, is an important step in understanding the long-term effects of these compounds on the environment. Constructed wetlands have been successfully utilized to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service