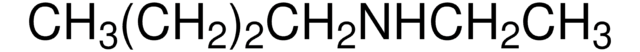All Photos(2)
About This Item
Linear Formula:
CH3(CH2)4NHCH3
CAS Number:
Molecular Weight:
101.19
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
Assay
98%
refractive index
n20/D 1.41 (lit.)
bp
116-118 °C (lit.)
density
0.738 g/mL at 25 °C (lit.)
SMILES string
CCCCCNC
InChI
1S/C6H15N/c1-3-4-5-6-7-2/h7H,3-6H2,1-2H3
InChI key
UOIWOHLIGKIYFE-UHFFFAOYSA-N
Application
N-Methylpentylamine can be used as an organic structure-directing agent in the preparation of microporous silicoaluminophosphate (SAPO) molecular sieves.
It can also be used as a reactant to synthesize:
It can also be used as a reactant to synthesize:
- N
- ′-tert-butyl-N-methyl-N-pentylsulfamide by reacting with triethylammonium N-tert-butylsulfamate in the presence of triphenylphosphine oxide and trifluoromethanesulfonic anhydride.
- 1-methyl-3-(methylpentylamino)-4-(phenylseleno) 1H-pyrrole-2,5-dione (aminoarylselenated maleimide) by Cu-catalyzed four-component coupling reaction with methyl maleimide, Se powder and iodobenzene.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Flam. Liq. 2 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
55.0 °F - closed cup
Flash Point(C)
12.77 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A novel approach for facilitating the targeted synthesis of silicoaluminophosphates
Yan Nana, et al.
Journal of Material Chemistry A, 6(47), 24186-24193 (2018)
Xue Gao et al.
Organic letters, 21(3), 745-748 (2019-01-15)
The first example of copper-catalyzed four-component coupling reaction of aryl iodides, Se powder, secondary amines, and maleimides is developed. This reaction provides an efficient and concise route to access aminoarylselenated maleimides via double C-Se bonds and C-N bond formation. The
Interactions of alkylamines with the silicon (001) surface.
Cao X and Hamers RJ.
J. Vac. Sci. Technol. B, 20(4), 1614-1619 (2002)
Efficient synthesis of unsymmetrical sulfamides from sulfamic acid salts by activation with triphenylphosphine ditriflate
Shehata MF, et al.
Tetrahedron, 75(24), 3186-3194 (2019)
Oliver Wahl et al.
Journal of pharmaceutical and biomedical analysis, 114, 254-264 (2015-06-21)
The modern bisphosphonate drug ibandronate sodium, a challenging candidate for impurity profiling, was analyzed using high performance liquid chromatography (HPLC) combined with corona charged aerosol detection (CAD). Separation was achieved on a mixed mode column combining hydrophobic C18 and strong
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service