286338
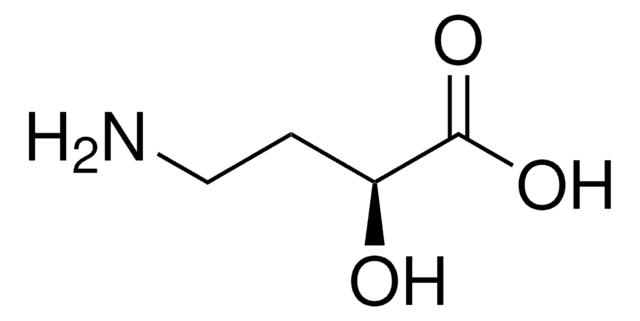
DL-Isoserine
98%
Synonym(s):
(±)-3-Amino-2-hydroxypropionic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
H2NCH2CH(OH)CO2H
CAS Number:
Molecular Weight:
105.09
Beilstein:
1721413
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
powder
reaction suitability
reaction type: solution phase peptide synthesis
mp
235 °C (dec.) (lit.)
application(s)
peptide synthesis
SMILES string
NCC(O)C(O)=O
InChI
1S/C3H7NO3/c4-1-2(5)3(6)7/h2,5H,1,4H2,(H,6,7)
InChI key
BMYNFMYTOJXKLE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Jan Cz Dobrowolski et al.
Physical chemistry chemical physics : PCCP, 12(36), 10818-10830 (2010-07-10)
The IR low-temperature Ar and Kr matrix spectra of l-isoserine were registered for the first time and interpreted by means of the anharmonic DFT frequencies calculated at the B3LYP/aug-cc-pVTZ and B3LYP/aug-cc-pVDZ levels. 54 l-isoserine conformers were predicted to be stable
Zhong-Xing Jiang et al.
The Journal of organic chemistry, 69(16), 5486-5489 (2004-08-04)
A novel and efficient enantioselective synthesis of both enantiomers of syn-(3-trifluoromethyl)isoserine was achieved. Ring opening of trifluoromethylated cyclic sulfates 3, derived from enantiopure trifluoromethylated vicinal diols 2, with various nucleophiles occurred exclusively at C2 with inversion of chirality. Treatment of
Alexander Titz et al.
Bioorganic & medicinal chemistry, 18(1), 19-27 (2009-12-02)
The selectin-leukocyte interaction is the initial event in the early inflammatory cascade. This interplay proceeds via the terminal tetrasaccharide sialyl Lewis(x) (sLe(x)), present on physiological selectin ligands and E- and P-selectins located on the endothelial surface. Blocking this process is
Nagarjuna Palyam et al.
The Journal of organic chemistry, 74(11), 4390-4392 (2009-05-02)
We report a new protocol for synthesis of L-1-deoxymannojirimycin, L-1-deoxyidonojirimycin, and the N-isopropyl derivative of the latter compound from the readily available precursors (S)-isoserinal hydrate and 2-tert-butyl-2-methyl-1,3-dioxan-5-one. The key steps include diastereoselective proline-catalyzed syn aldol transformation and a reductive amination/cyclization.
B S Coller et al.
The Journal of biological chemistry, 268(28), 20741-20743 (1993-10-05)
Peptides containing sequences derived from the new NH2 terminus of the seven-transmembrane domain thrombin receptor after thrombin cleavage can activate platelets directly. We recently demonstrated that such peptides are readily cleaved and inactivated by plasma, serum, and endothelial cell-associated aminopeptidase
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service