144436
Isobutyramide
99%
Synonym(s):
2-Methylpropionamide
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
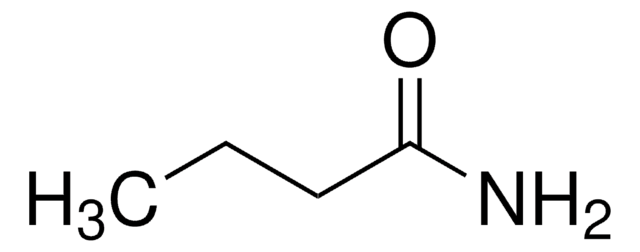
(CH3)2CHCONH2
CAS Number:
Molecular Weight:
87.12
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
solid
bp
216-220 °C (lit.)
mp
127-131 °C (lit.)
density
1.013 g/mL at 25 °C (lit.)
functional group
amide
SMILES string
CC(C)C(N)=O
InChI
1S/C4H9NO/c1-3(2)4(5)6/h3H,1-2H3,(H2,5,6)
InChI key
WFKAJVHLWXSISD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Isobutyramide was used for chemical grafting of human serum albumin during the synthesis of sequentialy assembled protein capsules.
Biochem/physiol Actions
Isobutyramide activates transcription of human gamma-globin gene and murine embryonic epsilon(y)-globin gene. It is useful in the treatment of β-thalassemia and sickle cell disease.
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M E Gleave et al.
Journal of cellular biochemistry, 69(3), 271-281 (1998-05-15)
Progression to androgen independence remains the main obstacle to improving survival and quality of life in patients with advanced prostate cancer. Induction of differentiation may serve as a rational basis for prevention of progression to androgen independence by modulating gene
Thermosensitive molecular assemblies from poly(amidoamine) dendron-based lipids.
Kenji Kono et al.
Angewandte Chemie (International ed. in English), 50(28), 6332-6336 (2011-05-21)
S Reich et al.
Blood, 96(10), 3357-3363 (2000-11-09)
The butyrate derivative isobutyramide (IBT) increases fetal hemoglobin (HbF) in patients with beta-hemoglobinopathies, but little is known about its usefulness for prolonged therapeutic use. We treated 8 patients with transfusion-dependent beta-thalassemia with 350 mg/kg of body weight per day of
G B Strambini et al.
Biochemistry, 29(1), 203-208 (1990-01-09)
The phosphorescence properties of liver alcohol dehydrogenase from horse were characterized at limiting concentrations of coenzyme and coenzyme analogues. The emission decay kinetics of Trp-314 in strong, slowly exchanging, ternary complexes with NADH/isobutyramide, NAD/pyrazole, and NADH/dimethyl sulfoxide displays a markedly
J Zhang et al.
Chinese medical sciences journal = Chung-kuo i hsueh k'o hsueh tsa chih, 16(4), 187-193 (2003-08-09)
To examine the effect of isobutyramide synthesized in our laboratory on human and murine globin gene expression and to test cell toxicity of the drug. MEL cells were transfected with the recombinant construct muLCRAgammapsibetadeltabeta and the stable transformants were cultured
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service