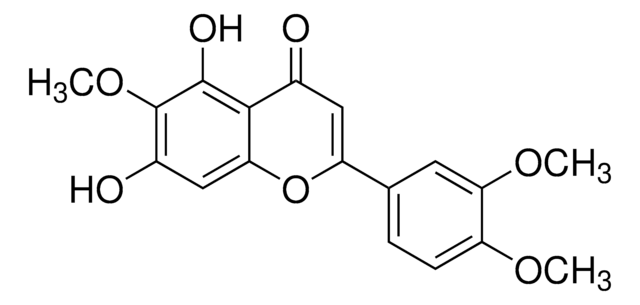
SML3743
Tegobuvir
≥98% (HPLC)
别名:
GS 9190, 5-({6-[2,4-Bis(trifluoromethyl)phenyl]pyridazin-3-yl}methyl)-2-(2-fluorophenyl)-5H-imidazo[4,5-c]pyridine, 5-[[6-[2,4-Bis(trifluoromethyl)phenyl]-3-pyridazinyl]methyl]-2-(2-fluorophenyl)-5H-imidazo[4,5-c]pyridine, GS 333126, GS-333126, GS-9190, GS333126, GS9190, TGV
登录查看公司和协议定价
所有图片(1)
About This Item
经验公式(希尔记法):
C25H14F7N5
CAS号:
分子量:
517.40
MDL號碼:
分類程式碼代碼:
51111800
分類程式碼代碼:
12352200
NACRES:
NA.77
推荐产品
生化/生理作用
Tegobuvir (GS-9190; TGV) is an orally active, non-cytotoxic imidazopyridine that, upon CYP-mediated intracellular activation, forms a glutathione conjugate that acts as a covalent, potent and genotype 1 replicons-selective non-nucleoside inhibitor (NNI) against hepatitis C virus (HCV) NS5B RNA-dependent RNA polymerase (IC50 = 0.8 nM/1b, 13.8 nM/1a, 21.9 μM/2a, >50 μM/1a; IC50 >100 nM against 2b, 3a, 4a, 5a, and 6a). When combined with suboptimal concentrations of other direct acting antivirals (DAAs) in cultures, tegobuvir (6-30 nM) is highly effective in curing cells from HCV replicon and in delaying or preventing the development of resistance against other DAAs.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Christy M Hebner et al.
PloS one, 7(6), e39163-e39163 (2012-06-22)
Tegobuvir (TGV) is a novel non-nucleoside inhibitor (NNI) of HCV RNA replication with demonstrated antiviral activity in patients with genotype 1 chronic HCV infection. The mechanism of action of TGV has not been clearly defined despite the identification of resistance
Tegobuvir (GS-9190) potency against HCV chimeric replicons derived from consensus NS5B sequences from genotypes 2b, 3a, 4a, 5a, and 6a.
Virology, 429(1), 57-62 (2012)
Mechanistic characterization of GS-9190 (Tegobuvir), a novel nonnucleoside inhibitor of hepatitis C virus NS5B polymerase
Antimicrobial Agents and Chemotherapy, 55(9), 4196-4203 (2011)
Inge Vliegen et al.
Antiviral research, 120, 112-121 (2015-06-04)
Tegobuvir (GS-9190) is a non-nucleoside inhibitor of HCV RNA replication with proven antiviral activity in HCV-infected patients. The in vitro antiviral activity of Tegobuvir, when combined with one or two other direct acting antivirals (DAA) was assessed. When Tegobuvir was
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门