推荐产品
product name
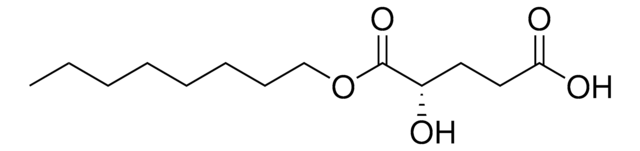
辛-(R)-2HG, ≥98% (HPLC)
化驗
≥98% (HPLC)
形狀
film
儲存條件
desiccated
顏色
colorless
溶解度
DMSO: 2 mg/mL, clear
儲存溫度
−20°C
應用
辛基-(R)-2羟基戊二酸酯(HG)已被用作胶质母细胞瘤细胞中的膜渗透性致癌代谢物(oncometabolite),用于测试其对NANOG转录因子表达的影响。它还被用作酮戊二酸酯(α-KG)依赖性脱氧酶α的竞争性抑制剂。
生化/生理作用
辛基-(R)-2HG (Octyl-D-2HG) 是肿瘤细胞因 NADP + 依赖性异柠檬酸脱氢酶基因 IDH1 和 IDH2 突变而产生的肿瘤代谢产物 D-2-羟基戊二酸盐 (D-2HG) 的膜渗透前体形式。D-2HG 通过与 α-KG 结合竞争抑制多种 α-酮戊二酸/α-KG-依赖性双加氧酶。通过 Octyl-(R)-2HG 处理 (1-50 mM) 的细胞 D-2HG 传递显示出抑制脱甲基酶活性(~148%H3K9me2 和 ~310%H3K79me2 上调;U-87 mg 中 50 mm)以及因为分别抑制 α-KG-依赖性双加氧酶脯氨酰羟化酶 (PHD) 和胶原脯氨酰-4-羟化酶 (C-P4H)而增加 HIF-1α通过, 降低内皮抑素水平。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
Zachary J Reitman et al.
The Journal of biological chemistry, 289(34), 23318-23328 (2014-07-06)
Mutations in the cytosolic NADP(+)-dependent isocitrate dehydrogenase (IDH1) occur in several types of cancer, and altered cellular metabolism associated with IDH1 mutations presents unique therapeutic opportunities. By altering IDH1, these mutations target a critical step in reductive glutamine metabolism, the
alpha-Ketoglutarate-Activated NF-$\kappa$B Signaling Promotes Compensatory Glucose Uptake and Brain Tumor Development
Wang X, et al.
Molecular Cell, 76(1), 148-162 (2019)
IDH1R132H Causes Resistance to HDAC Inhibitors by Increasing NANOG in Glioblastoma Cells
Kim G H, et al.
International Journal of Molecular Sciences, 20(11), 2679-2679 (2019)
Jing-Yi Chen et al.
Scientific reports, 6, 32428-32428 (2016-09-01)
Mutations of isocitrate dehydrogenase 1 (IDH1) and IDH2 in acute myeloid leukemia (AML) cells produce the oncometabolite R-2-hydroxyglutarate (R-2HG) to induce epigenetic alteration and block hematopoietic differentiation. However, the effect of R-2HG released by IDH-mutated AML cells on the bone
Parker L Sulkowski et al.
Science translational medicine, 9(375) (2017-02-06)
2-Hydroxyglutarate (2HG) exists as two enantiomers, (R)-2HG and (S)-2HG, and both are implicated in tumor progression via their inhibitory effects on α-ketoglutarate (αKG)-dependent dioxygenases. The former is an oncometabolite that is induced by the neomorphic activity conferred by isocitrate dehydrogenase
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门