推荐产品
化驗
≥98% (HPLC)
形狀
powder
光學活性
[α]/D ≥+225°, c = 1 in H2O
顏色
white to tan
溶解度
H2O: ≥18 mg/mL
儲存溫度
2-8°C
SMILES 字串
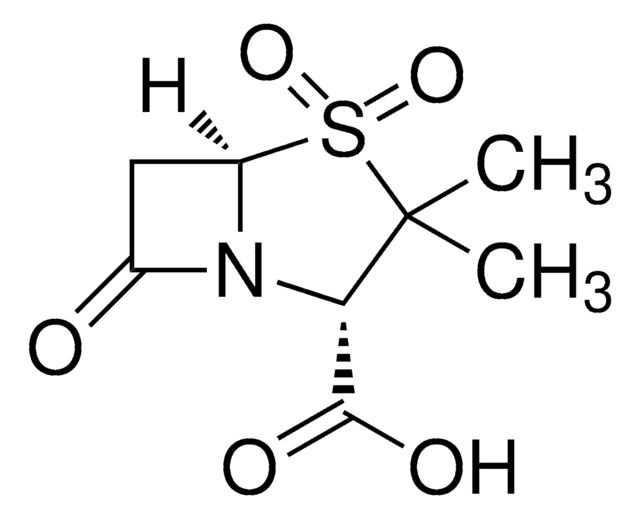
CC1(C)[C@@H](N2[C@@H](CC2=O)S1(=O)=O)C(O)=O
InChI
1S/C8H11NO5S/c1-8(2)6(7(11)12)9-4(10)3-5(9)15(8,13)14/h5-6H,3H2,1-2H3,(H,11,12)/t5-,6+/m1/s1
InChI 密鑰
FKENQMMABCRJMK-RITPCOANSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
舒巴坦是一种半合成青霉素砜,来源于6-氨基青霉烷酸。这种β-内酰胺化合物的特征为具有一个 β-内酰胺环。
應用
舒巴坦可用作β-内酰胺酶抑制剂测试中的β-内酰胺酶抑制剂。 它也可用于评估其对极端耐药的鲍曼不动杆菌的抗菌药效学作用。舒巴坦可用于细胞信号转导研究。
生化/生理作用
舒巴坦为 β-内酰胺酶的不可逆抑制剂,可使β-内酰胺如青霉素和头孢菌素失活。它也可对拟杆菌和革兰氏阴性菌的某些染色体介导酶起作用。它具有有限的抗菌活性。 但是,舒巴坦与其它潜在抗生素的联合使用对多重耐药型鲍曼不动杆菌感染有治疗作用。
訊號詞
Danger
危險聲明
危險分類
Resp. Sens. 1 - Skin Sens. 1
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
Mallika Sengupta et al.
Cureus, 14(2), e21802-e21802 (2022-03-08)
Background Acinetobacter species are known to be important hospital-acquired pathogens. Unfortunately, multidrug-resistant Acinetobacter spp. has very limited options for an effective treatment. Aim To identify the common pathogens causing lower respiratory tract infections (LRTI), their antimicrobial susceptibility pattern, and determine the minimum inhibitory
Sarah M Drawz et al.
Clinical microbiology reviews, 23(1), 160-201 (2010-01-13)
Since the introduction of penicillin, beta-lactam antibiotics have been the antimicrobial agents of choice. Unfortunately, the efficacy of these life-saving antibiotics is significantly threatened by bacterial beta-lactamases. beta-Lactamases are now responsible for resistance to penicillins, extended-spectrum cephalosporins, monobactams, and carbapenems.
Krisztina M Papp-Wallace et al.
Antimicrobial agents and chemotherapy, 56(11), 5687-5692 (2012-08-22)
Acinetobacter baumannii is an increasingly problematic pathogen in United States hospitals. Antibiotics that can treat A. baumannii are becoming more limited. Little is known about the contributions of penicillin binding proteins (PBPs), the target of β-lactam antibiotics, to β-lactam-sulbactam susceptibility
A S Levin
Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases, 8(3), 144-153 (2002-05-16)
Recent studies have highlighted the emergence of infections involving multiresistant Acinetobacter clinical isolates. Sulbactam offers direct antimicrobial activity against Acinetobacter species. Accordingly, co-administration of sulbactam with ampicillin or cefoperazone offers the potential of effective empirical therapy against Acinetobacter and other
Mei Li et al.
Antimicrobial agents and chemotherapy, 56(11), 5678-5686 (2012-08-22)
Ambler position 105 in class A β-lactamases is implicated in resistance to clavulanic acid, although no clinical isolates with mutations at this site have been reported. We hypothesized that Y105 is important in resistance to clavulanic acid because changes in
商品
Bioactive small molecules for immune system signaling target identification/validation and antibiotics, antivirals, and antifungals offered.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门