推荐产品
品質等級
化驗
≥98% (HPLC)
形狀
powder
溶解度
methanol: 1 mg/mL, clear, colorless
應用
metabolomics
vitamins, nutraceuticals, and natural products
儲存溫度
2-8°C
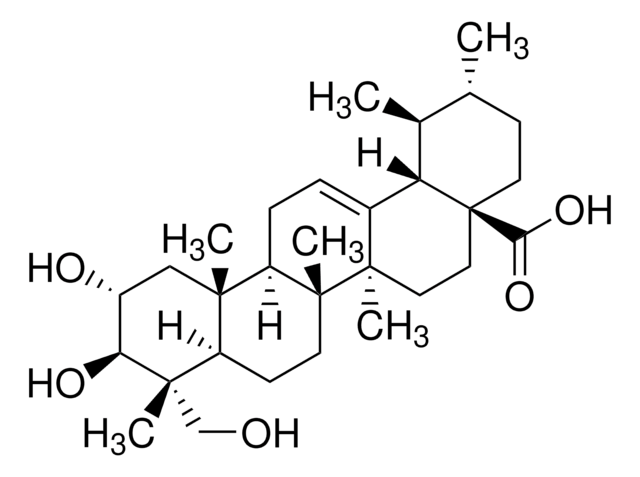
SMILES 字串
O[C@H]1[C@H](O)C(C)(C)[C@@](CC[C@]2(C)[C@]3([H])CC=C4[C@@]2(C)CC[C@]5(C(O)=O)[C@@]4([H])[C@@H](C)[C@H](C)CC5)([H])[C@]3(C)C1
InChI
1S/C30H48O4/c1-17-10-13-30(25(33)34)15-14-28(6)19(23(30)18(17)2)8-9-22-27(5)16-20(31)24(32)26(3,4)21(27)11-12-29(22,28)7/h8,17-18,20-24,31-32H,9-16H2,1-7H3,(H,33,34)/t17-,18+,20-,21+,22-,23+,24+,27+,28-,29-,30+/m1/s1
InChI 密鑰
HFGSQOYIOKBQOW-ZSDYHTTISA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
Corosolic acid from Lagerstroemia speciosa is a pentacyclic triterpenoid found in a variety of plants, such as apples, basil, bilberries, cranberries, and prunes. It is a natural derivative of ursolic acid.
應用
Corosolic acid from Lagerstroemia speciosa has been used to study the inhibiting activity of ursolic acid and its derivative against colon cancer cells by causing degradation of β-catenins.
生化/生理作用
Corosolic acid from Lagerstroemia speciose shows a variety of biological activities such as anti-proliferation, apoptosis, and anti-carcinogenic. It exhibits an inhibiting effect on the post-challenge plasma glucose levels in humans. Corosolic acid, due to its ability to improve glucose metabolism, has been touted to be used in the management of Type-2 Diabetes. It improves hypertension, oxidative stress, and inflammation, thereby being a potential candidate to be used against atherosclerosis-related diseases.
Triterpene phytochemical found in medicinal herbal extracts. Possesses antiatherosclerotic, antihyperlipidemic, antioxidant, antiinflammatory, antifungal, antiviral and antineoplastic activities.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Yanfeng Xu et al.
Cancer letters, 284(2), 229-237 (2009-05-22)
We investigated the response of human cervix adenocarcinoma HeLa cells to Corosolic acid (CRA) treatment. Our results showed that CRA significantly inhibited cell viability in both a dose- and a time-dependent manner. CRA treatment induced S cell-cycle arrest and caused
M Fukushima et al.
Diabetes research and clinical practice, 73(2), 174-177 (2006-03-22)
Corosolic acid (CRA) is a substance extracted from Lagerstroemia speciosa L. and has been reported to have biological activities in in vitro and experimental animal studies. In this study, 31 subjects were orally administered 10mg CRA or a placebo, on
G Sivakumar et al.
Current medicinal chemistry, 18(1), 79-90 (2010-11-30)
Globally, diabetes and obesity are two of the most common metabolic diseases of the 21(st) century. Increasingly, not only adults but children and adolescents are being affected. New approaches are needed to prevent and treat these disorders and to reduce
Myung Sun Lee et al.
Phytotherapy research : PTR, 24(1), 49-53 (2009-06-24)
Four ursane-type triterpenoids, corosolic acid (1), ilekudinol B (2), ursolic acid (3) and pomolic acid (4), were isolated from an EtOAc-soluble extract of the leaves of Weigela subsessilis. These bioactive compounds were evaluated for their glucose uptake activity and produced
Wenli Hou et al.
Phytotherapy research : PTR, 23(5), 614-618 (2008-12-25)
The potential antidiabetic activity of ethyl acetate extract of the leaves of Lagerstroemia speciosa (LSL) was investigated by alpha-amylase and alpha-glucosidase inhibition assay. Six pentacyclic triterpenes (oleanolic acid, arjunolic acid, asiatic acid, maslinic acid, corosolic acid and 23-hydroxyursolic acid) were
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门