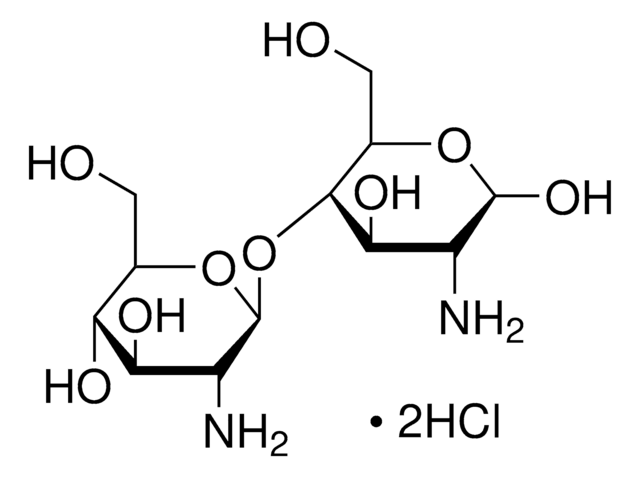
D1523
N,N′-Diacetylchitobiose
≥96% (HPLC)
别名:
2-Acetamido-2-deoxy-4-O-(2-acetamido-2-deoxy-β-D-glucopyranosyl)-D-glucopyranose, 4-O-(2-Acetamido-2-deoxy-β-D-glucopyranosyl)-2-acetamido-2-deoxy-D-glucose, Chitobiose
About This Item
推荐产品
品質等級
化驗
≥96% (HPLC)
形狀
powder
光學活性
[α]/D 15.00 to 19.00 °, c = 9.00-11.00 mg/mL in water
顏色
off-white
mp
245-247 °C (lit.)
溶解度
H2O: 49.00-51.00 mg/mL, clear, colorless
儲存溫度
−20°C
SMILES 字串
CC(=O)N[C@H]1C(O)O[C@H](CO)[C@@H](O[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2NC(C)=O)[C@@H]1O
InChI
1S/C16H28N2O11/c1-5(21)17-9-13(25)14(8(4-20)27-15(9)26)29-16-10(18-6(2)22)12(24)11(23)7(3-19)28-16/h7-16,19-20,23-26H,3-4H2,1-2H3,(H,17,21)(H,18,22)/t7-,8-,9-,10-,11-,12-,13-,14-,15?,16+/m1/s1
InChI 密鑰
CDOJPCSDOXYJJF-CBTAGEKQSA-N
正在寻找类似产品? 访问 产品对比指南
應用
生化/生理作用
準備報告
其他說明
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门