推荐产品
形狀
powder
品質等級
起源
Eli Lilly
儲存溫度
2-8°C
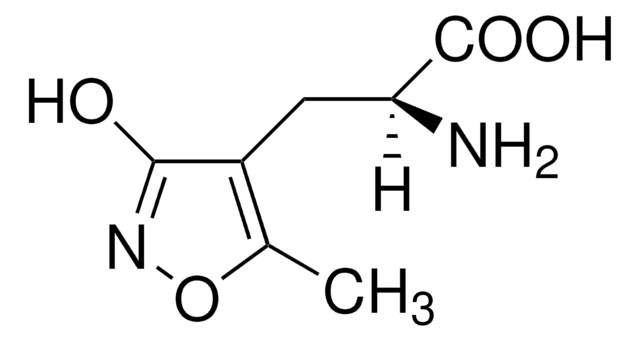
SMILES 字串
[H][C@@]12CC(C3Nc4cc(Cl)c(cc4S(=O)(=O)N3)S(N)(=O)=O)[C@@]([H])(C1)C=C2
InChI
1S/C14H16ClN3O4S2/c15-10-5-11-13(6-12(10)23(16,19)20)24(21,22)18-14(17-11)9-4-7-1-2-8(9)3-7/h1-2,5-9,14,17-18H,3-4H2,(H2,16,19,20)/t7-,8+,9?,14?/m0/s1
InChI 密鑰
BOCUKUHCLICSIY-QJWLJZLASA-N
基因資訊
human ... CA1(759) , CA4(762) , GRIA1(2890) , GRIA2(2891) , GRIA3(2892) , GRIA4(2893)
rat ... Gria1(50592)
正在寻找类似产品? 访问 产品对比指南
一般說明
Cyclothiazide is a benzothiadiazine, which has a similar structure to diazoxide.
應用
Cyclothiazide has been used as a α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) desensitization blocker to study the effects of γ2 on receptor desensitization.
生化/生理作用
Blocks the rapid desensitization of the AMPA glutamate receptors and markedly prolongs the decay time of the evoked excitatory post-synaptic current.
特點和優勢
This compound is a featured product for Neuroscience research. Click here to discover more featured Neuroscience products. Learn more about bioactive small molecules for other areas of research at sigma.com/discover-bsm.
This compound is featured on the Glutamate Receptors (Ion Channel Family) page of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
This compound was developed by Eli Lilly. To browse the list of other pharma-developed compounds and Approved Drugs/Drug Candidates, click here.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Helle Hald et al.
Journal of molecular biology, 391(5), 906-917 (2009-07-14)
Ionotropic glutamate receptors (iGluRs) mediate fast excitatory neurotransmission. Upon glutamate application, 2-amino-3-(3-hydroxy-5-methyl-4-isoxazolyl)propionic acid receptors undergo rapid and almost complete desensitization that can be attenuated by positive allosteric modulators. The molecular mechanism of positive allosteric modulation has been elucidated previously by
A novel Conus snail polypeptide causes excitotoxicity by blocking desensitization of AMPA receptors.
Craig S Walker et al.
Current biology : CB, 19(11), 900-908 (2009-06-02)
Ionotropic glutamate receptors (iGluRs) are glutamate-gated ion channels that mediate excitatory neurotransmission in the central nervous system. Based on both molecular and pharmacological criteria, iGluRs have been divided into two major classes, the non-NMDA class, which includes both AMPA and
L O Trussell et al.
Neuron, 10(6), 1185-1196 (1993-06-01)
We have investigated the role of AMPA receptor desensitization during transmission at a calyceal synapse. Cyclothiazide blocked the rapid desensitization of AMPA receptors and markedly prolonged the decay time of the evoked excitatory postsynaptic current (PSC). This effect was greater
Lisa Grant et al.
The Journal of neuroscience : the official journal of the Society for Neuroscience, 30(12), 4210-4220 (2010-03-26)
Cochlear inner hair cells (IHCs) convert sounds into receptor potentials and via their ribbon synapses into firing rates in auditory nerve fibers. Multivesicular release at individual IHC ribbon synapses activates AMPA-mediated EPSCs with widely ranging amplitudes. The underlying mechanisms and
Autumn M Weeks et al.
Neuropharmacology, 85, 57-66 (2014-06-01)
Positive allosteric modulators of α-amino-3-hydroxy-5-methyl-isoxazole-propionic acid (AMPA) ionotropic glutamate receptors facilitate synaptic plasticity and contribute essentially to learning and memory, properties which make AMPA receptors targets for drug discovery and development. One region at which several different classes of positive
商品
DISCOVER Bioactive Small Molecules for Neuroscience
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门