推荐产品
化驗
≥98% (HPLC)
形狀
solid
顏色
off-white
溶解度
DMSO: soluble 10 mg/mL
起源
GlaxoSmithKline
儲存溫度
−20°C
SMILES 字串
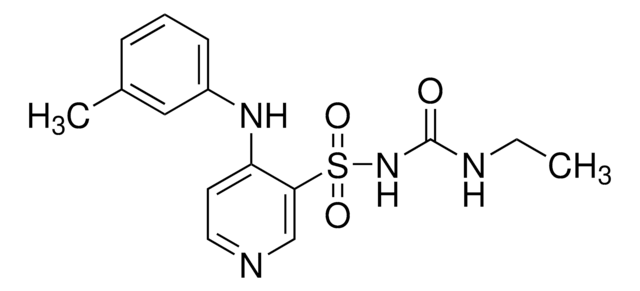
O[C@H](CCN1[C@@H](CCCCCCC(O)=O)C(=O)NC1=O)C2CCCCC2
InChI
1S/C19H32N2O5/c22-16(14-8-4-3-5-9-14)12-13-21-15(18(25)20-19(21)26)10-6-1-2-7-11-17(23)24/h14-16,22H,1-13H2,(H,23,24)(H,20,25,26)/t15-,16+/m0/s1
InChI 密鑰
ZIDQIOZJEJFMOH-JKSUJKDBSA-N
基因資訊
human ... PTGDR(5729)
生化/生理作用
BW 245C was investigated for the affinities potencies, and intrinsic activities in comparison with other natural and synthetic prostanoids using endogenous receptors. The rank order of compound affinities at the DP receptor was SQ27986 (K(i) = 10 +/- 2 nM) > RS93520 = ZK110841 = BW245C (K(i) = 23-26 nM) > ZK118182 (K(i) = 50 +/- 9 nM) > PGD(2) (K(i) = 80 +/- 5 nM). DP receptor agonists produced cAMP in embryonic bovine tracheal fibroblasts with different potencies (EC(50) values in nM): ZK118182 (18 +/- 6), RS93520 (28 +/- 6), SQ27986 (29 +/- 7), ZK110841 (31 +/- 7), BW245C (53 +/- 16), and PGD(2) (98 +/- 10). BW245C was more efficacious and RS93520 was less efficacious.
特點和優勢
This compound is featured on the Prostanoid Receptors page of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
This compound was developed by GlaxoSmithKline. To browse the list of other pharma-developed compounds and Approved Drugs/Drug Candidates, click here.
儲存類別代碼
13 - Non Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
Xibin Liang et al.
Journal of neurochemistry, 92(3), 477-486 (2005-01-22)
Cyclo-oxygenases (COXs) catalyze the first committed step in the synthesis of the prostaglandins PGE(2), PGD(2), PGF(2alpha), PGI(2) and thomboxane A(2). Expression and enzymatic activity of COX-2, the inducible isoform of COX, are observed in several neurological diseases and result in
N A Sharif et al.
The Journal of pharmacology and experimental therapeutics, 293(2), 321-328 (2000-04-25)
The prostanoid receptor-subtype binding affinities, selectivities, potencies, and intrinsic activities of four natural prostanoids and six synthetic DP class prostanoids were determined using binding and functional assays with endogenous receptors. SQ27986 exhibited the highest affinity for the human platelet DP
Alicja Jozkowicz et al.
Antioxidants & redox signaling, 10(12), 2035-2046 (2008-07-31)
15-Deoxy-Delta(12,14)-prostaglandin-J(2) (15d-PGJ(2)) is a cyclopentenone prostaglandin regarded as antiinflammatory mediator, which can act through peroxisome proliferator-activated receptor-gamma (PPARgamma) or through G protein-coupled surface receptors. It has been demonstrated that 15d-PGJ(2) potently increases the generation of interleukin-8 (IL-8) in human microvascular
C Yoshimura-Uchiyama et al.
Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology, 34(8), 1283-1290 (2004-08-10)
Both prostaglandin (PG) D receptor (DP) and CRTH2 (chemoattractant receptor-homologous molecule expressed on Th2 cells)/DP2 are high-affinity receptors for PGD2. Previous studies have demonstrated that PGD2 enhances releasability and induces CRTH2/DP2-mediated migration in human basophils, but the precise effects of
Sybille van den Brule et al.
The Journal of pharmacology and experimental therapeutics, 335(2), 472-479 (2010-08-20)
Prostaglandin (PG) D(2) exerts contrasting activities in the inflamed lung via two receptors, the D prostanoid receptor (DP) and the chemoattractant receptor-homologous molecule expressed on T helper 2 lymphocytes. DP activation is known mainly to inhibit proinflammatory cell functions. We
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门