所有图片(1)
About This Item
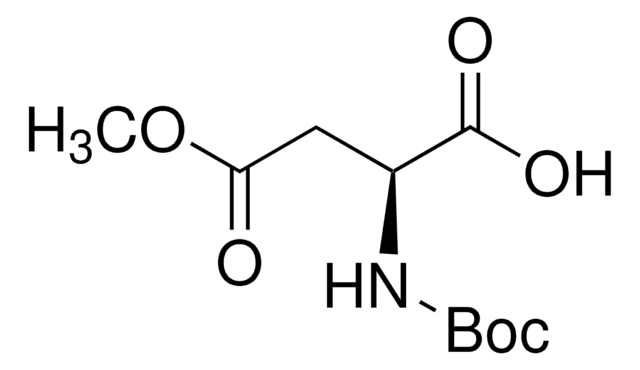
经验公式(希尔记法):
C5H9NO4 · HCl
CAS号:
分子量:
183.59
EC號碼:
MDL號碼:
分類程式碼代碼:
12352200
PubChem物質ID:
NACRES:
NA.26
推荐产品
化驗
≥98%
形狀
powder
顏色
white
儲存溫度
−20°C
SMILES 字串
Cl.COC(=O)C[C@H](N)C(O)=O
InChI
1S/C5H9NO4.ClH/c1-10-4(7)2-3(6)5(8)9;/h3H,2,6H2,1H3,(H,8,9);1H/t3-;/m0./s1
InChI 密鑰
QRBMPUYOGOCYDJ-DFWYDOINSA-N
生化/生理作用
L-Aspartic acid β-methyl ester is used for the organic synthesis of S-enantiomers of hanishin, longamide B, and longamide B methylester, of 1-β-methylcarbapenem, TA-949 and for the enzymatic synthesis of a CCK-4 tripeptide fragment
儲存類別代碼
13 - Non Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
Jignesh Patel et al.
The Journal of organic chemistry, 70(22), 9081-9084 (2005-10-22)
[reaction: see text] Total syntheses of enantiopure hanishin, longamide B, and longamide B methyl ester are described. Absolute configurations of these natural products have been established.
Li Guo et al.
Di 1 jun yi da xue xue bao = Academic journal of the first medical college of PLA, 23(4), 289-292 (2003-04-17)
To synthesize a tripeptide derivative Phac-Met-Asp(OMe)-Phe -NH2, which is a fragment of the gastrin C-terminal tetrapeptide CCK-4, by enzymatic reaction. Three free enzymes, alpha-chymotrypsin, papain and thermolysin from acyl donor Phac-Met-OCam was involved in three steps. The choice of appropriate
M Seki et al.
The Journal of organic chemistry, 65(2), 517-522 (2000-05-18)
A facile and economical synthesis of a novel orally active 1-beta-methylcarbapenem, TA-949 (1), is described. The key process involves an efficient synthesis of the C-2 side chain (R)-4-mercaptopyrrolidine-2-thione 2 from L-aspartic acid and the construction of the 1-beta-methylcarbapenem skeleton. The
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门