推荐产品
生物源
synthetic
品質等級
化驗
≥96.0% (TLC)
形狀
powder
官能基
carboxylic acid
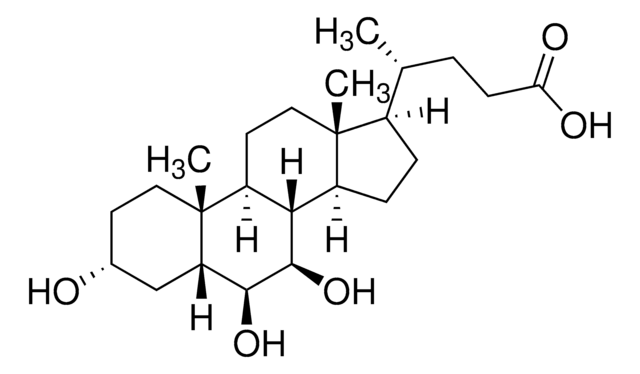
SMILES 字串
[H][C@@]12[C@]([C@](CC[C@@H](O)C3)(C)[C@]3([H])C[C@@H]2O)([H])CC[C@@]4(C)[C@@]1([H])CC[C@]4([H])[C@]([H])(C)CCC(NCC(O)=O)=O
InChI
1S/C26H43NO5/c1-15(4-7-22(30)27-14-23(31)32)18-5-6-19-24-20(9-11-26(18,19)3)25(2)10-8-17(28)12-16(25)13-21(24)29/h15-21,24,28-29H,4-14H2,1-3H3,(H,27,30)(H,31,32)/t15-,16+,17-,18-,19+,20+,21+,24+,25+,26-/m1/s1
InChI 密鑰
GHCZAUBVMUEKKP-XROMFQGDSA-N
應用
- Glycoursodeoxycholic Acid Alleviates Arterial Thrombosis via Suppressing Diacylglycerol Kinases Activity in Platelet.: Highlights the therapeutic potential of Glycoursodeoxycholic acid in alleviating arterial thrombosis by inhibiting diacylglycerol kinase activity in platelets (Yang et al., 2024).
生化/生理作用
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
实验方案
Investigate bile acid roles in gut hormone profiles and glycemic control, vital for clinical labs exploring potential mechanisms.
相关内容
Bile Acids (BA) are synthesized in the liver and play important roles in cholesterol homeostasis, absorption of vitamins and lipids, and various key metabolic processes.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门