所有图片(1)
About This Item
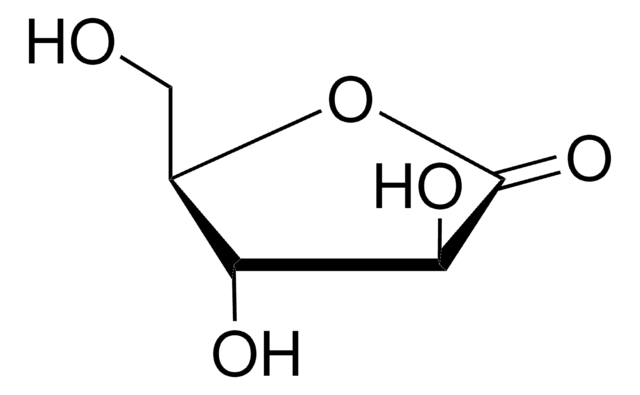
经验公式(希尔记法):
C6H10O6
CAS号:
分子量:
178.14
Beilstein:
83005
MDL號碼:
分類程式碼代碼:
12352201
PubChem物質ID:
NACRES:
NA.25
推荐产品
化驗
≥95.0% (GC)
形狀
solid
光學活性
[α]/D 78.0±3.0°, c = 1 in H2O
顏色
white
mp
134 °C ((273 °F))
適合性
conforms to structure for Proton NMR spectrum
儲存溫度
−20°C
SMILES 字串
OC[C@H](O)[C@H]1OC(=O)[C@@H](O)[C@@H]1O
InChI
1S/C6H10O6/c7-1-2(8)5-3(9)4(10)6(11)12-5/h2-5,7-10H,1H2/t2-,3-,4-,5+/m0/s1
InChI 密鑰
SXZYCXMUPBBULW-NEEWWZBLSA-N
正在寻找类似产品? 访问 产品对比指南
相关类别
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Rosalia Liso et al.
Journal of experimental botany, 55(408), 2589-2597 (2004-11-03)
To understand the function of ascorbic acid (ASC) in root development, the distribution of ASC, ASC oxidase, and glutathione (GSH) were investigated in cells and tissues of the root apex of Cucubita maxima. ASC was regularly distributed in the cytosol
Peter Schertl et al.
The Journal of biological chemistry, 287(18), 14412-14419 (2012-03-02)
L-galactono-1,4-lactone dehydrogenase (GLDH) catalyzes the terminal step of the Smirnoff-Wheeler pathway for vitamin C (l-ascorbate) biosynthesis in plants. A GLDH in gel activity assay was developed to biochemically investigate GLDH localization in plant mitochondria. It previously has been shown that
Joanna Maddison et al.
Planta, 214(3), 383-391 (2002-02-22)
Leaf L-ascorbate content of an ozone (O3)-sensitive radish genotype (Raphanus sativus L. cv. Cherry Belle) was increased 2-fold by feeding hydroponically cultivated plants L-galactono- 1,4-lactone (GalL). Plants were grown in controlled-environment chambers ventilated with charcoal/Purafil-filtered air, and administered one of
Claudio Stasolla et al.
Plant physiology and biochemistry : PPB, 45(3-4), 188-198 (2007-04-03)
In previous studies we have reported that applications of ascorbic acid (ACS) enhance the conversion frequency of white spruce somatic embryos by "rescuing" structurally disorganized meristems and inducing cell proliferation in the apical poles [C. Stasolla, E.C. Yeung, Ascorbic acid
Md Moinuddin Ahmed et al.
Carbohydrate research, 341(10), 1505-1521 (2006-04-18)
A short and highly efficient route to various C-4 substituted sugar lactones has been developed. The key to the overall transformation is the sequential osmium-catalyzed dihydroxylation reaction of substituted 2,4-dienoates and an allylic substitution at the C-4 position. When the
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门