推荐产品
生物源
synthetic
等級
pharmaceutical primary standard
agency
EP
API 家族
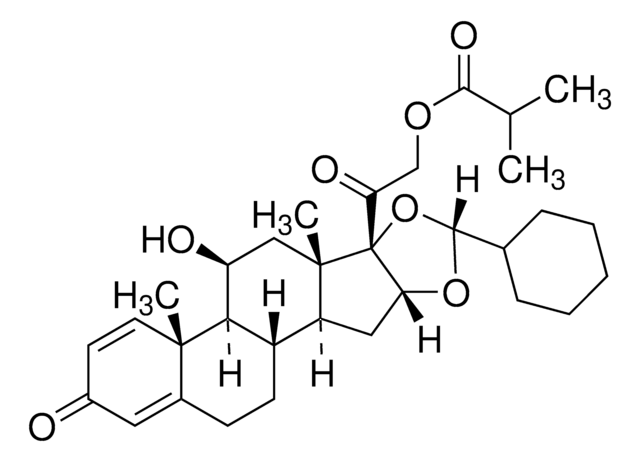
ciclesonide
包裝
pkg of 150 mg
製造商/商標名
EDQM
儲存條件
protect from light
顏色
white
bp
665.0 °C/1.333 hPa (1229.0°F)
mp
209-211 °C (408—412°F)
溶解度
water: <0.1 g/L
acetone: soluble
ethanol: soluble
密度
1.23 g/cm3 at 20 °C (1.333 hPa)
應用
pharmaceutical (small molecule)
格式
neat
運輸包裝
ambient
儲存溫度
2-8°C
SMILES 字串
C[C@@]12C(CC[C@]3([H])[C@]2([H])[C@@H](O)C[C@@]4(C)[C@@]3([H])C[C@]5([H])[C@]4(O[C@@](C6CCCCC6)([H])O5)C(COC(C(C)C)=O)=O)=CC(C=C1)=O
InChI
1S/C32H44O7/c1-18(2)28(36)37-17-25(35)32-26(38-29(39-32)19-8-6-5-7-9-19)15-23-22-11-10-20-14-21(33)12-13-30(20,3)27(22)24(34)16-31(23,32)4/h12-14,18-19,22-24,26-27,29,34H,5-11,15-17H2,1-4H3/t22-,23-,24-,26+,27+,29+,30-,31-,32+/m0/s1
InChI 密鑰
LUKZNWIVRBCLON-GXOBDPJESA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets, have been developed and issued under the Authority of the issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
應用
包裝
其他說明
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门