推荐产品
等級
pharmaceutical primary standard
API 家族
bisoprolol
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
格式
neat
儲存溫度
2-8°C
SMILES 字串
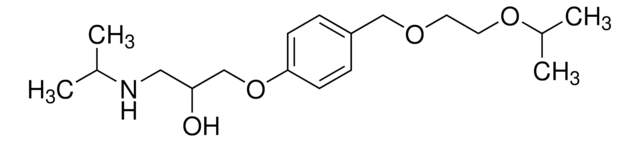
OC(=O)\C=C\C(O)=O.CC(C)NCC(O)COc1ccc(COCCOC(C)C)cc1.CC(C)NCC(O)COc2ccc(COCCOC(C)C)cc2
InChI
1S/2C18H31NO4.C4H4O4/c2*1-14(2)19-11-17(20)13-23-18-7-5-16(6-8-18)12-21-9-10-22-15(3)4;5-3(6)1-2-4(7)8/h2*5-8,14-15,17,19-20H,9-13H2,1-4H3;1-2H,(H,5,6)(H,7,8)/b;;2-1+
InChI 密鑰
VMDFASMUILANOL-WXXKFALUSA-N
基因資訊
human ... ADRB1(153)
正在寻找类似产品? 访问 产品对比指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Bisoprolol fumarate EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
生化/生理作用
心脏选择性 β1-肾上腺素受体拮抗剂。
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
相關產品
产品编号
说明
价格
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral - Repr. 2 - STOT RE 2
標靶器官
Heart
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Akira Sezai et al.
The Journal of thoracic and cardiovascular surgery, 144(5), 1241-1248 (2012-08-04)
We previously performed a trial of intravenous landiolol hydrochloride during and after cardiac surgery (the PASCAL trial) and demonstrated a preventive effect on postoperative atrial fibrillation (AF). In the present study, we investigated the efficacy of increasing the dose and
Viktor Nagy
Orvosi hetilap, 154(44), 1731-1734 (2013-10-29)
The prevalence of chronic heart failure in Hungary is 1.6% in the adult population, but it occurs in 15-20% of subjects over 80 years of age. The base of treatment of heart failure is the blockade of the neuro-hormonal system
Benjamin J Hirsh et al.
Circulation. Heart failure, 5(5), 560-565 (2012-08-03)
Chronotropic incompetence is defined as the inability to reach 80% of heart rate (HR) reserve or 80% of the maximally predicted HR during exercise. The presence of chronotropic incompetence is associated with reduced peak oxygen consumption, and rate-responsive pacing therapy
János Tomcsányi et al.
Orvosi hetilap, 154(7), 267-271 (2013-02-12)
The authors describe two cases of takotsubo cardiomyopathy developing after an abrupt withdrawal of carvedilol and bisoprolol. Takotsubo or stress cardiomyopathy is characterized by acute and reversible cardiac dysfunction without coronary artery disease. It is triggered by acute emotional or
Huichao Chang et al.
Journal of pharmaceutical and biomedical analysis, 71, 104-110 (2012-09-06)
A sensitive, specific liquid chromatography-tandem mass spectrometry (LC-MS/MS) method was established for the quantitative determination of amlodipine and bisoprolol, using clenbuterol as the internal standard (IS). The analytes and IS were isolated from 100μL plasma samples by a simple liquid-liquid
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门