推荐产品
等級
pharmaceutical primary standard
API 家族
valnemulin
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
格式
neat
儲存溫度
2-8°C
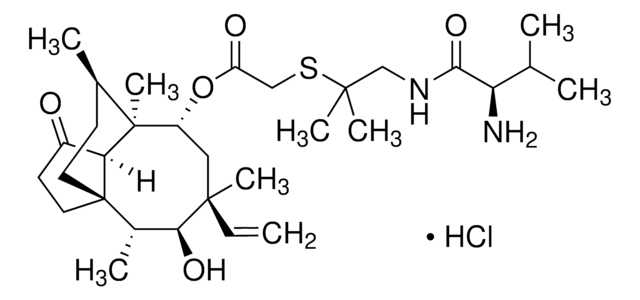
SMILES 字串
C[C@@H]1CC[C@@]23CCC(=O)[C@H]2[C@]1(C)[C@@H](C[C@@](C)(C=C)[C@@H](O)[C@@H]3C)OC(=O)CO
InChI
1S/C22H34O5/c1-6-20(4)11-16(27-17(25)12-23)21(5)13(2)7-9-22(14(3)19(20)26)10-8-15(24)18(21)22/h6,13-14,16,18-19,23,26H,1,7-12H2,2-5H3/t13-,14+,16-,18+,19+,20-,21+,22+/m1/s1
InChI 密鑰
ZRZNJUXESFHSIO-BKUNHTPHSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
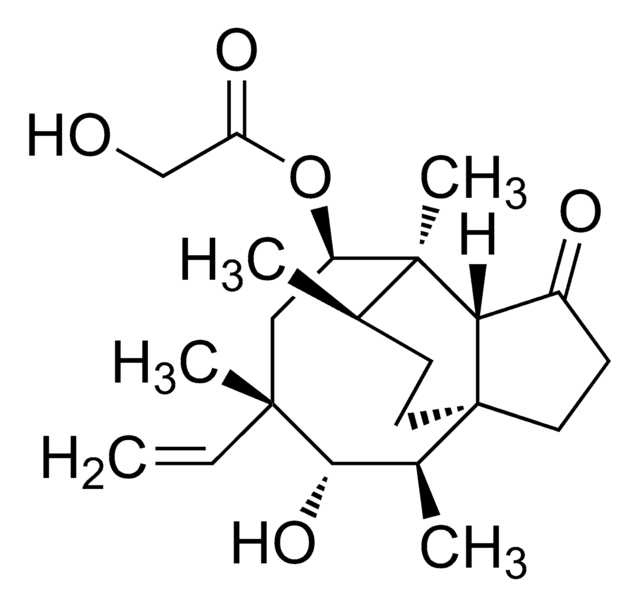
Valnemulin impurity E EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
生化/生理作用
Pleuromutilin is an antibiotic natural product that inhibits bacterial protein synthesis by binding to bacterial ribosomes in the peptidyl transferase center and inhibiting peptide bond formation.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Rodger Novak
Annals of the New York Academy of Sciences, 1241, 71-81 (2011-12-24)
In 1951, the first reference to the antibacterial substance pleuromutilin was made in a paper published in the Proceedings of the National Academy of Sciences. Researchers had identified several species of the mold genus Pleurotus that inhibited the growth of
Ruofeng Shang et al.
Journal of medicinal chemistry, 57(13), 5664-5678 (2014-06-04)
A series of novel pleuromutilin derivatives possessing thiadiazole moieties were synthesized via acylation reactions under mild conditions. The in vitro antibacterial activities of the derivatives against methicillin-resistant Staphylococcus aureus, methicillin-resistant Staphylococcus epidermidis, Escherichia coli, and Streptococcus agalactiae were tested by
Rodger Novak et al.
Current opinion in investigational drugs (London, England : 2000), 11(2), 182-191 (2010-01-30)
Pleuromutilins were discovered as natural-product antibiotics in 1950. Tiamulin was the first pleuromutilin compound to be approved for veterinary use in 1979, followed by valnemulin in 1999. It was not until 2007 that retapamulin became the first pleuromutilin approved for
Y-Z Tang et al.
Mini reviews in medicinal chemistry, 12(1), 53-61 (2011-11-11)
Due to the rapid onset of resistance to most antibacterial drugs, research efforts are focusing on new classes of antibacterials with different mechanisms of action from clinically used antibacterials. Pleuromutilin derivatives have received more and more scientific attention for their
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门