所有图片(1)
About This Item
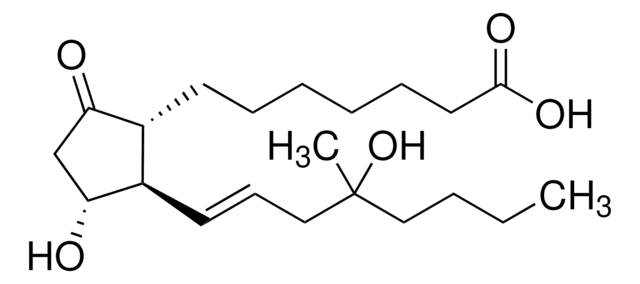
经验公式(希尔记法):
C22H38O5
CAS号:
分子量:
382.53
MDL號碼:
分類程式碼代碼:
41116107
PubChem物質ID:
NACRES:
NA.24
推荐产品
等級
pharmaceutical primary standard
API 家族
misoprostol
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
格式
neat
儲存溫度
−20°C
SMILES 字串
CCCCC(C)(O)C\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1CCCCCCC(=O)OC
InChI
1S/C22H38O5/c1-4-5-14-22(2,26)15-10-12-18-17(19(23)16-20(18)24)11-8-6-7-9-13-21(25)27-3/h10,12,17-18,20,24,26H,4-9,11,13-16H2,1-3H3/b12-10+/t17-,18-,20-,22?/m1/s1
InChI 密鑰
OJLOPKGSLYJEMD-URPKTTJQSA-N
基因資訊
human ... PTGER3(5733)
正在寻找类似产品? 访问 产品对比指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Misoprostol EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
生化/生理作用
PGE 1 类似物前体药物,迅速去酯化为活性“米索前列醇酸”。广泛的治疗作用,包括预防 NSAID 诱导的胃溃疡、调节免疫级联反应、抑制血小板活化因子(PAF)、治疗乙醇和扑热息痛诱导的肝毒性和肝炎以及刺激损伤后的软骨修复。
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral - Repr. 1B
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Deborah Bartz et al.
Obstetrics and gynecology, 122(1), 57-63 (2013-06-08)
To compare the efficacy and acceptability of buccal misoprostol or a synthetic osmotic cervical dilator for cervical preparation before same-day late first-trimester and early second-trimester surgical abortion. In this randomized, double-blind trial, we compared 400 micrograms of buccal misoprostol with
Jeffrey Michael Smith et al.
BMC pregnancy and childbirth, 13, 44-44 (2013-02-21)
Hemorrhage continues to be a leading cause of maternal death in developing countries. The 2012 World Health Organization guidelines for the prevention and management of postpartum hemorrhage (PPH) recommend oral administration of misoprostol by community health workers (CHWs). However, there
C W Ho et al.
Alimentary pharmacology & therapeutics, 37(8), 819-824 (2013-02-26)
Poor adherence to gastroprotective agents (GPAs) is common among users of nonsteroidal anti-inflammatory drugs (NSAIDs) or low-dose aspirin (ASA). There are little data on the utilization of GPAs among NSAID and ASA users complicated by ulcer bleeding. To study the
Heleen J van Beekhuizen et al.
International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics, 122(3), 234-237 (2013-06-25)
To evaluate the efficacy and safety of misoprostol among patients with retained placenta in a low-resource setting. A prospective, multicenter, randomized, double-blind, placebo-controlled trial was carried out in Tanzania between April 2008 and November 2011. It included patients who delivered
Amanda Selk et al.
Obstetrics and gynecology, 118(4), 941-949 (2011-09-22)
To estimate the benefits and harms of misoprostol use for cervical dilation in patients undergoing operative hysteroscopy. We searched MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials (from inception to February 2011). We also searched trial registries, other
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门