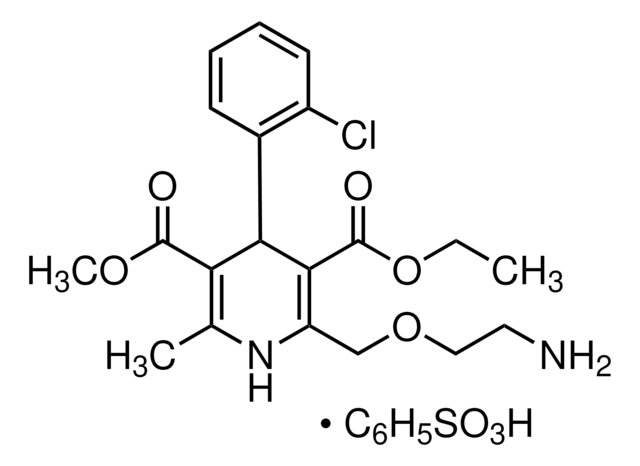
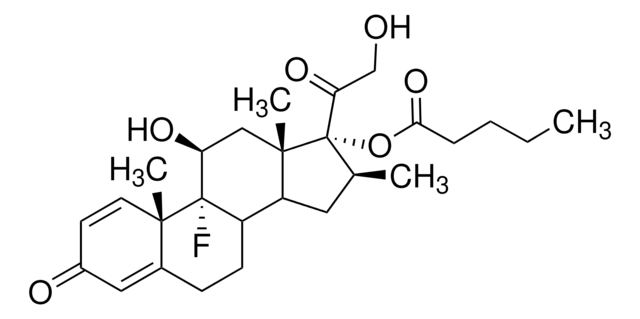
推荐产品
生物源
synthetic
品質等級
等級
certified reference material
pharmaceutical secondary standard
agency
traceable to BP BP041
traceable to Ph. Eur. B1045000
traceable to USP 1068004
描述
Pharmaceutical Secondary Standard; Certified Reference Material
形狀
solid
包裝
pkg of 1000 mg
應用
pharmaceutical small molecule
儲存溫度
2-8°C
一般說明
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
應用
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
分析報告
These secondary standards offer multi-traceability to the USP, EP and BP primary standards, where they are available.
其他說明
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
腳註
To see an example of a Certificate of Analysis for this material enter LRAA6959(changes by product) in the documents slot below. This is an example certificate only and may not be the lot that you receive.
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 4 Oral - Repr. 1B - STOT RE 2
標靶器官
Liver,Kidney,Endocrine system
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Choose from one of the most recent versions:
分析证书(COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门