推荐产品
等級
analytical standard
品質等級
化驗
≥95.0% (HPLC)
儲存期限
limited shelf life, expiry date on the label
技術
HPLC: suitable
gas chromatography (GC): suitable
應用
food and beverages
格式
neat
SMILES 字串
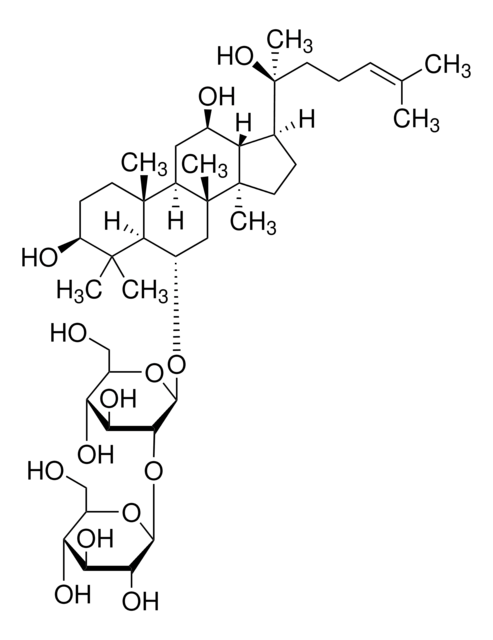
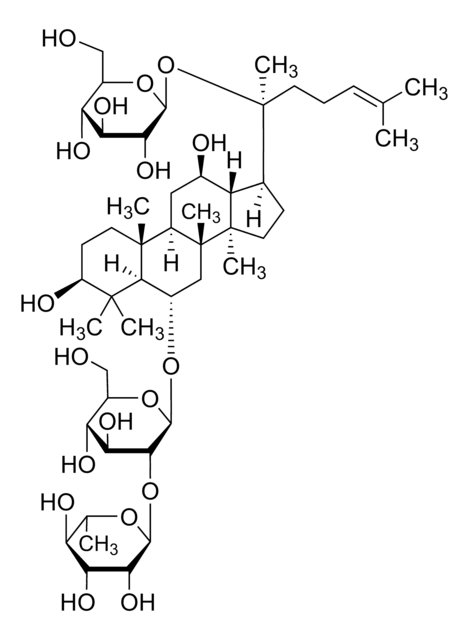
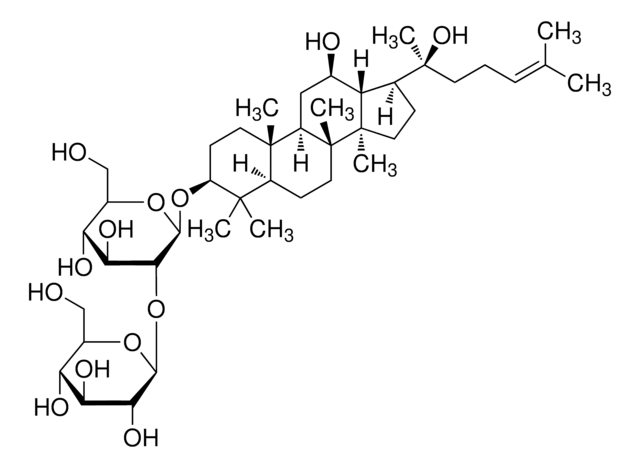
[H][C@]1(O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O)O[C@@H]2[C@@H](O)[C@H](O)[C@@H](CO)O[C@@]2([H])O[C@H]3C[C@]4(C)[C@]([H])(C[C@@H](O)[C@]5([H])[C@]([H])(CC[C@@]45C)[C@@](C)(O)CC\C=C(/C)C)[C@@]6(C)CC[C@H](O)C(C)(C)[C@]36[H]
InChI
1S/C42H72O14/c1-20(2)10-9-13-42(8,52)21-11-15-40(6)28(21)22(45)16-26-39(5)14-12-27(46)38(3,4)35(39)23(17-41(26,40)7)53-37-34(32(50)30(48)25(19-44)55-37)56-36-33(51)31(49)29(47)24(18-43)54-36/h10,21-37,43-52H,9,11-19H2,1-8H3/t21-,22+,23-,24+,25+,26+,27-,28-,29+,30+,31-,32-,33+,34+,35-,36-,37+,39+,40+,41+,42-/m0/s1
InChI 密鑰
UZIOUZHBUYLDHW-XUBRWZAZSA-N
正在寻找类似产品? 访问 产品对比指南
應用
其他說明
其他客户在看
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门