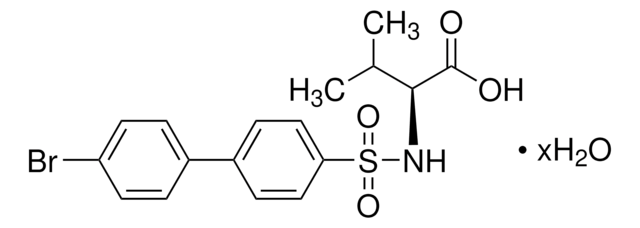
5.32283
CXCR2 Antagonist IV, Sch527123
别名:
CXCR2 Antagonist IV, Sch527123, 2-Hydroxy-N,N-dimethyl-3-((2-((1( R)-(5-methyl-2-furanyl)propyl)amino)-3,4-dioxo-1-cyclobuten-1-yl)amino)benzamide, ( R)-2-Hydroxy-N,N-dimethyl-3-(2-(1-(5-methylfuran-2-yl)propylamino)-3,4-dioxocyclobut-1-enylamino)benzamide, CXCR1 Antagoni
登录查看公司和协议定价
所有图片(1)
About This Item
推荐产品
化驗
≥97% (HPLC)
品質等級
形狀
solid
製造商/商標名
Calbiochem®
儲存條件
OK to freeze
protect from light
顏色
yellow
溶解度
DMSO: 50 mg/mL
儲存溫度
2-8°C
InChI
1S/C21H23N3O5/c1-5-13(15-10-9-11(2)29-15)22-16-17(20(27)19(16)26)23-14-8-6-7-12(18(14)25)21(28)24(3)4/h6-10,13,22-23,25H,5H2,1-4H3/t13-/m1/s1
InChI 密鑰
RXIUEIPPLAFSDF-CYBMUJFWSA-N
一般說明
A cell-permeable orally available (plasma Cmax/AUC/dose = 17.0 µM/31.5 µM h-10-24 h/25 mg kg-1/mouse; 5.0 µM/23.0 µM h-10-48 h/10 mg kg-1/rat; 0.9 µM/4.0 µM h-10-48 h/3 mg kg-1/Cynomolgus monkey) cyclobutenedione compound that targets CXCR2 intracellular allosteric site via high affinity interaction (Kd = 49 pM/human, 80 pM/Cynomolgus monkey, 200 pM/ mouse & rat) in a reversible and slowly dissociating manner (k2 = 0.00058 min-1; t1/2 ≈ 22 h; human CXCR2), while exhibiting much reduced affinity toward CXCR1 (Kd = 3.9 nM/human & 41 nM/Cynomolgus monkey) due to a much faster dissociation rate (k2 = 0.141 min-1; t1/2 ≈ 5 min; human CXCR1) and displaying no significant efficacy toward a large panel of GPCRs, enzymes, and ion channels (IC50 >10 µM), including CXCR3 & CCR5. Likewise, Sch527123 is shown exhibit much higher potency against CXCR2- than CXCR1-mediated chemotaxis of Ba/F3 transfectants (IC50/ligand/CXCR2 species = 2.8 nM/3 nM hCXCL8/monkey, 3.4 nM/3 nM rCINC-3/rat, 5.6 nM/mCXCR2/3 nM mMIP-2, <<3 nM/0.1 to 60 nM hCXCL8/human; % inhibition/[Sch527123]/ligand/CXCR1 species = 35%/300 nM/3 nM hCXCL8/ human & 50%/9.667 µM/3 nM hCXCL8/monkey) without affecting C5a- or fMLP-induced human neutrophil chemotaxis. Oral administration is shown to effectively reduce pulmonary neutrophilia in vivo among LPS- or V2O5-treated mice & rats (ED50 1.3 to 1.8 mg/kg), as well as among Cynomolgus monkeys receiving repeat bronchoscopy & lung lavage (ED50 0.3 mg/kg). Also reported to inhibit hCXCL8-dependent human melanoma A375SM chemotaxis & invasion (79% & 69% inhibition, respectively, with 10 ng/mL = 25 nM Sch527123; overnight at 37 °C;10 ng/mL hCXCL8) and effectively suppress A375SM proliferation both in cultures in vitro (31% inhibition by MTT assay post 72 h treatment with 1 µg/mL = 2.5 µM Sch527123) and in mice in vivo (79% inhibition on D50 post cancer cells injection; 100 mg/kg/d p.o. D1 through D22) with concomitant increased apoptotic cells (203% of control) and decreased microvessel density in tumor tissues (55% of control).
A cell-permeable, orally active, non-toxic, cyclobutenedione compound with anti-inflammatory properties. Acts as a specific, high-affinity, and potent allosteric antagonist of CXCR2 (IC50 = 2.6 nM for human; Kd = 49, 200, and 80 pM for human, rat, and cynomolgus, respectively). Also exhibits high potency against CXCR1 (IC50 = 36 nM for human, Kd = 3.9 and 41 nM for human and cynomolgus, respectively). Displays excellent selectivity over CXCR3, CCR5, and a large panel of GPCRs, enzymes and ion channels (~ 10 µM). Potently inhibits CXCL1- and CXCL8-induced chemotaxis in human neutrophils (hPMN; IC50<1 nM and 16 nM, respectively). Shown to suppress pulmonary neutrophilia (ED50 = 1.3 mg/kg), goblet cell hyperplasia (ED50 = 700 mg/kg), and increase bronchoalveolar lavage (BAL) mucin content (ED50 ≤ 1 mg/kg) in rats subjected to vanadium pentoxide induced pulmonary inflammation. Inhibits melanoma cell proliferation (~ 1 mg/ml) by suppressing the phosphorylation of ERK1/2. Diminishes the invasive potential of melanoma cells and blocks angiogenesis in mice (100 mg/kg, p.o., q.d for 21 days). Exhibits desirable pharmacokinetic properties.
Please note that the molecular weight for this compound is batch-specific due to variable water content.
Please note that the molecular weight for this compound is batch-specific due to variable water content.
生化/生理作用
Cell permeable: yes
Primary Target
CXCR2
CXCR2
Reversible: yes
Secondary Target
CXCR1
CXCR1
包裝
Packaged under inert gas
警告
Toxicity: Standard Handling (A)
重構
Following reconstitution, aliquot and freeze (-20°C). Stock solutions are stable for up to 6 months at -20°C.
Use only fresh DMSO for reconstitution.
其他說明
Seiberling, M., et al. 2013. Int. Immunopharmacol.17, 178.
Salchow, K., et al. 2010. Br. J. Pharmacol.159, 1429.
Singh, S., et al. 2009. Clin. Cancer Res.15, 2380.
Chapman, R.W., et al. 2007. J. Pharmacol. Exp. Ther.322, 486.
Gonsiorek, W., et al. 2007. J. Pharmacol. Exp. Ther.322, 477.
Dwyer, M.P., et al. 2006. J. Med. Chem.49, 7603.
Salchow, K., et al. 2010. Br. J. Pharmacol.159, 1429.
Singh, S., et al. 2009. Clin. Cancer Res.15, 2380.
Chapman, R.W., et al. 2007. J. Pharmacol. Exp. Ther.322, 486.
Gonsiorek, W., et al. 2007. J. Pharmacol. Exp. Ther.322, 477.
Dwyer, M.P., et al. 2006. J. Med. Chem.49, 7603.
法律資訊
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门