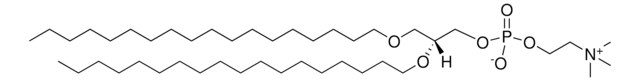
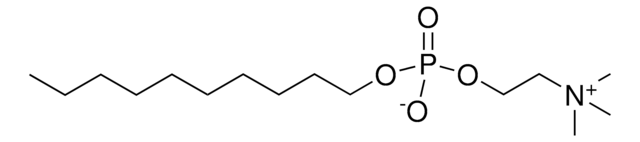
推荐产品
品質等級
化驗
≥98% (TLC)
形狀
crystalline solid
製造商/商標名
Calbiochem®
儲存條件
OK to freeze
protect from light
溶解度
ethanol: 1 mg/mL
PBS, pH 7.2: 2.5 mg/mL
DMSO: 800 μg/mL
運輸包裝
ambient
儲存溫度
−20°C
InChI
1S/C21H46NO4P/c1-5-6-7-8-9-10-11-12-13-14-15-16-17-18-20-25-27(23,24)26-21-19-22(2,3)4/h5-21H2,1-4H3
InChI 密鑰
PQLXHQMOHUQAKB-UHFFFAOYSA-N
一般說明
An ether lipid analog that acts as an inhibitor of CTP:phosphocholine cytidyltransferase and displays remarkable antiproliferative effect both in vitro and in vivo. Also induces apoptosis mediated by cell-permeable ceramides.
An ether lipid analog that acts as an inhibitor of CTP:phosphocholine cytidyltransferase. Reported to exhibit anti-proliferative and anti-metastatic effects in vitro and in vivo. Also reported to induce apoptosis mediated by cell-permeable ceramides. Shown to have anti-protozoal activity against Leishmania species.
生化/生理作用
Cell permeable: no
Primary Target
CTP:phosphocholine cytidyltransferase
CTP:phosphocholine cytidyltransferase
Product does not compete with ATP.
Reversible: no
包裝
Packaged under inert gas
警告
Toxicity: Harmful (C)
重構
Following reconstitution in ethanol or DMSO, aliquot and freeze (-20°C). Stock solutions in ethanol or DMSO are stable for up to 3 months at -20°C. Stock solutions in PBS are stable for up to 24 h at -20°C.
其他說明
Wieder, T., et al. 1998. J. Biol. Chem.273, 11025.
Wieder, T., et al. 1993. Biochem. J.291, 561.
Geilen, C.C., et al. 1992. J. Biol. Chem.267, 6719.
Geilen, C.C., et al. 1991. Eur. J. Cancer27, 1650.
Wieder, T., et al. 1993. Biochem. J.291, 561.
Geilen, C.C., et al. 1992. J. Biol. Chem.267, 6719.
Geilen, C.C., et al. 1991. Eur. J. Cancer27, 1650.
法律資訊
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
C C Geilen et al.
European journal of cancer (Oxford, England : 1990), 27(12), 1650-1653 (1991-01-01)
The antineoplastic agent, hexadecylphosphocholine, a phospholipid analogue, inhibited phosphatidylserine-activated protein kinase C in vitro at concentrations higher than 40 mumol/l. The half-inhibitory concentration (IC50) was 62 mumol/l. Another alkylphosphocholine, dodecylphosphocholine, did not have an inhibitory effect on protein kinase C.
T Wieder et al.
The Biochemical journal, 291 ( Pt 2), 561-567 (1993-04-15)
The antagonization of phorbol 12-myristate 13-acetate (PMA)-stimulated phosphatidylcholine (PtdCho) biosynthesis by the phospholipid analogue hexadecylphosphocholine (HePC) in MDCK cells was investigated and compared with the corresponding influence in HeLa cells. In both cell lines, PMA-stimulated PtdCho biosynthesis was antagonized by
C C Geilen et al.
The Journal of biological chemistry, 267(10), 6719-6724 (1992-04-05)
The mechanism of the inhibition of phosphatidylcholine biosynthesis by the phospholipid analogue, hexadecylphosphocholine, was investigated in Madin-Darby canine kidney cells. In the presence of 50 mumol/liter hexadecylphosphocholine, there was a translocation of CTP:choline-phosphate cytidylyltransferase (EC 22.7.7.15) activity from the membranes
Yash Gupta et al.
Journal of food and drug analysis, 30(1), 128-149 (2022-06-02)
Leishmaniasis remains a serious public health problem in many tropical regions of the world. Among neglected tropical diseases, the mortality rate of leishmaniasis is second only to malaria. All currently approved therapeutics have toxic side effects and face rapidly increasing
T Wieder et al.
The Journal of biological chemistry, 273(18), 11025-11031 (1998-06-06)
The prototype of a new class of antiproliferative phospholipid analogs, hexadecylphosphocholine (HePC), has been shown to inhibit tumor growth and is currently used for the treatment of cutaneous metastases of mammary carcinomas. Although several cellular targets of HePC, e.g. protein
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门