推荐产品
蒸汽密度
3.6 (vs air)
蒸汽壓力
9.75 mmHg ( 60 °C)
化驗
99%
反應適用性
reaction type: C-H Activation
bp
163-164 °C (lit.)
mp
32-35 °C (lit.)
密度
0.889 g/mL at 25 °C (lit.)
官能基
carboxylic acid
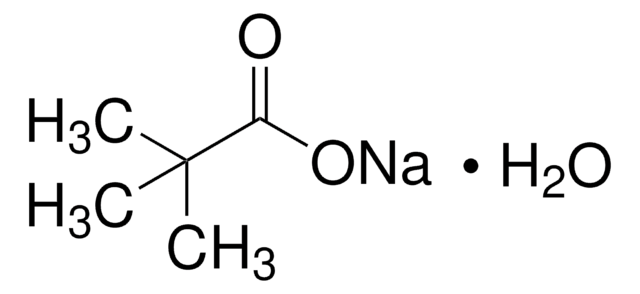
SMILES 字串
OC(C(C)(C)C)=O
InChI
1S/C5H10O2/c1-5(2,3)4(6)7/h1-3H3,(H,6,7)
InChI 密鑰
IUGYQRQAERSCNH-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
戊二酸是一种羧酸,用作配体以合成六核铈(IV)团簇。
應用
特戊酸可用于:
- 用作钯的助催化剂,芳化未活化芳烃和N-杂环化合物。
- 用作羰基化suzuki反应的添加剂,以纳米钯为催化剂从芳基碘和芳基硼酸合成二芳甲酮。
- 在8-氨基喹啉配体和钴催化剂作用下,参与苯甲酰胺与炔烃的环化反应,合成异喹诺酮类。
注意
刺激性臭味/恶臭
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 1
閃點(°F)
147.2 °F - closed cup
閃點(°C)
64 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Guotao Li et al.
Journal of the American Chemical Society, 130(12), 3740-3741 (2008-03-04)
Propargylic pivalates with electronically unbiased internal alkynes are selectively transformed into (1Z,3E)-2-pivaloxy-1,3-dienes containing various functionalities. The unusual selectivity of 1,2-acyloxy migration over the structurally preferred 3,3-rearrangement is realized. This reaction is highly stereoselective and offers rapid access to dienes for
Tobias Illg et al.
ChemSusChem, 4(3), 392-398 (2011-02-09)
The two-step synthesis of tert-butyl peroxypivalate is performed in a single-channel microreactor. The first step, the deprotonation of tert-butyl hydroperoxide, is done in a simple mixer tube setup. The residence time section for the second reaction step is equipped with
Toshiaki Shimasaki et al.
Angewandte Chemie (International ed. in English), 49(16), 2929-2932 (2010-03-17)
Catalytic amination: The title reaction demonstrates the use of aryl carboxylates as suitable electrophilic coupling substrates in catalytic amination reactions. N-heterocyclic carbene ligands and NaOtBu promote the amination of aryl pivalates through the cleavage of normally unreactive aryl carbon-oxygen bonds
Phosphine-free, palladium-catalyzed arylation of heterocycles through C-H bond activation with pivalic acid as a cocatalyst.
Dongbing Zhao et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 15(6), 1337-1340 (2008-12-31)
Lukas J Goossen et al.
Angewandte Chemie (International ed. in English), 48(20), 3569-3571 (2009-03-13)
Crucial breakthroughs in the activation of the C(aryl)-O bond of phenol derivatives were achieved almost simultaneously by two research groups (see scheme; Cy = cyclohexyl). Garg et al. coupled a range of aryl pivalates with arylboronic acids to give unsymmetrical
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门