推荐产品
化驗
98%
折射率
n20/D 1.559 (lit.)
bp
250 °C (lit.)
mp
2-5 °C (lit.)
密度
1.065 g/mL at 25 °C (lit.)
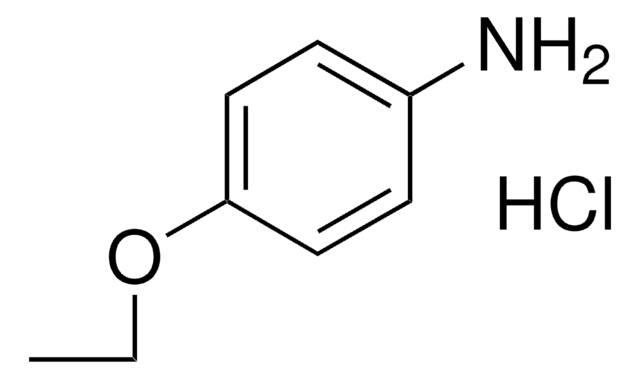
SMILES 字串
CCOc1ccc(N)cc1
InChI
1S/C8H11NO/c1-2-10-8-5-3-7(9)4-6-8/h3-6H,2,9H2,1H3
InChI 密鑰
IMPPGHMHELILKG-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
訊號詞
Warning
危險分類
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Muta. 2 - Skin Sens. 1
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 1
閃點(°F)
251.6 °F - closed cup
閃點(°C)
122 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
T Lindqvist et al.
Chemical research in toxicology, 4(4), 489-496 (1991-07-01)
4-Ethoxyaniline (p-phenetidine) is oxidized by peroxidases to form several products, one of which is 4-[(4-ethoxyphenyl)imino]-2,5-cyclohexadien-1-one (1). This compound reacts with N-acetylcysteine (NAC) in methanol-phosphate buffers, generating at least four different products. Four major products, 4-[(4-ethoxyphenyl)amino]phenol (2), 3-(N-acetylcystein-S-yl)-4-[(4-ethoxyphenyl)amino]phenol (3), 2,5-bis(N-acetylcystein-S-yl)-4-[(4-ethoxyphenyl)-amino]phenol (4)
Annabel Kuek et al.
Postgraduate medical journal, 83(978), 251-260 (2007-04-04)
Targeted biologic therapies have revolutionised treatment of immune-mediated inflammatory diseases (IMIDs) due to their efficacy, speed of onset and tolerability. The discovery that clinically unrelated conditions, such as rheumatoid arthritis and Crohn's disease, share similar immune dysregulation has led to
R Larsson et al.
The Journal of pharmacology and experimental therapeutics, 235(2), 475-480 (1985-11-01)
The metabolism of p-phenetidine in microsomes from rabbit kidney and the metabolism of acetaminophen and p-phenetidine in human kidney microsomes to protein binding metabolites were examined. Microsomal preparations from rabbit kidney medulla catalyzed the irreversible arachidonic acid-dependent binding of p-[14C]phenetidine
R Larsson et al.
Chemico-biological interactions, 60(3), 317-330 (1986-12-01)
The interaction of N-(4-ethoxyphenyl)p-benzoquinone imine (NEPBQI), a metabolite formed during peroxidase catalyzed metabolism of p-phenetidine, with GSH and its effects in isolated rat hepatocytes were investigated. When reacted with GSH NEPBQI formed both a mono- and a diglutathione conjugate as
J D Baty et al.
Journal of chromatography, 353, 329-337 (1986-02-26)
Liquid chromatographic methods were developed for the study of the in vitro acetylation of the sulphonamide drug sulphamethazine and a series of aniline derivatives. The sensitivity of the methods have allowed data on the activity of the N-acetyltransferase enzyme(s) in
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门