推荐产品
形狀
liquid
反應適用性
reaction type: Pegylations
reagent type: cross-linking reagent
折射率
n/D 1.4641
密度
1.1395 g/mL
官能基
Boc
amine
carboxylic acid
儲存溫度
−20°C
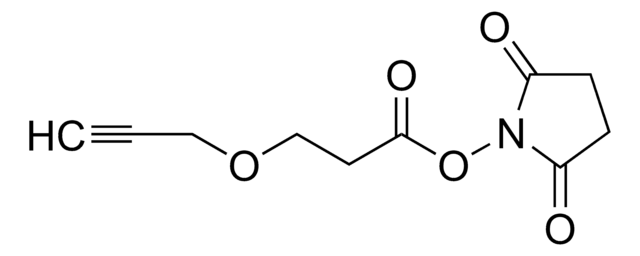
SMILES 字串
OC(COCCOCCOCCOCCNC(OC(C)(C)C)=O)=O
應用
This heterobifunctional, PEGylated crosslinker features a carboxylic acid at one end and Boc-protected amino group at the other, which can be deprotected with mildly acidic conditions. The hydrophilic PEG linker facilitates solubility in biological applications. BocNH-PEG4-acid can be used for bioconjugation or as a building block for synthesis of small molecules, conjugates of small molecules and/or biomolecules, or other tool compounds for chemical biology and medicinal chemistry that require ligation. Examples of applications include its synthetic incorporation into antibody-drug conjugates or proteolysis-targeting chimeras (PROTAC® molecules) for targeted protein degradation
Technology Spotlight: Degrader Building Blocks for Targeted Protein Degradation
Technology Spotlight: Degrader Building Blocks for Targeted Protein Degradation
其他說明
法律資訊
PROTAC is a registered trademark of Arvinas Operations, Inc., and is used under license
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
A biocompatible condensation reaction for the labeling of terminal cysteine residues on proteins.
Hongjun Ren et al.
Angewandte Chemie (International ed. in English), 48(51), 9658-9662 (2009-11-20)
BisPNA targeting to DNA: Effect of neutral loop on DNA duplex strand invasion by aepPNA-N7GlaepPNA-C substituted peptide nucleic acids
Shirude P S, et sl.
European Journal of Organic Chemistry, 2005, 5207-5215 (2005)
Jennifer L Brigham et al.
ACS chemical biology, 8(4), 691-699 (2013-01-12)
Bioorthogonal ligation methods that allow the selective conjugation of fluorophores or biotin to proteins and small molecule probes that contain inert chemical handles are an important component of many chemical proteomic strategies. Here, we present a new catch-and-release enrichment strategy
Zinc(II) cyclen-peptide conjugates interacting with the weak effector binding state of RaS.
Rosnizeck I C, et al.
Inorgorganica Chimica Acta, 365 (1), 38-48 (2011)
Pratistha Ranjitkar et al.
Chemistry & biology, 17(2), 195-206 (2010-03-02)
A number of small-molecule inhibitors have been developed that target the catalytic domains of protein kinases that are not in an active conformation. An inactive form that has been observed in several kinases is the DFG-out conformation. This conformation is
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门