推荐产品
化驗
95%
形狀
liquid
折射率
n20/D 1.494
密度
1.146 g/mL at 25 °C
運輸包裝
wet ice
儲存溫度
2-8°C
SMILES 字串
C=CCOC(=O)n1ccnc1
InChI
1S/C7H8N2O2/c1-2-5-11-7(10)9-4-3-8-6-9/h2-4,6H,1,5H2
InChI 密鑰
NEFLGCHXJFBCQP-UHFFFAOYSA-N
一般說明
Allyl 1H-imidazole-1-carboxylate is an organic reagent used to introduce carboxyallyl groups to nucleophilic nitrogen, oxygen, and carbon centers. It is used in the acylation reactions of enolates and nitrogen compounds. Further, it can also be used in the synthesis of carbonates and allyl esters.
應用
Allyl 1H-imidazole-1-carboxylate can be used:
- To prepare allyl enol carbonate derivatives by reacting with ketone enolates and boron trifluoride etherate.
- In the acylation of a mixture of primary and secondary alcohols.
訊號詞
Warning
危險分類
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
A New Simple One-Pot Regioselective Preparation of Mixed Diesters of Carbonic Acid
Bertolini G, et al.
The Journal of Organic Chemistry, 63(17), 6031-6034 (1998)
Allyl 1H-imidazole-1-carboxylate
Encyclopedia of Reagents for Organic Synthesis, Second Edition (2015)
Barry M Trost et al.
The Journal of organic chemistry, 72(24), 9372-9375 (2007-10-30)
A convenient access to substituted allyl enol carbonates was established through the reaction of ketone enolates with the complex of allyl 1H-imidazole-1-carboxylates and boron trifluoride etherate.
相关内容
Professor Heller and coworkers are engaged in the development of mild and chemoselective acylation reactions using carbonylazole-derived reagents. To that end, they have developed a suite of carbonylimidazole derivatives for facile and chemoselective esterification (MImC, etc.) and amidation (WImC) of carboxylic acids, in collaboration with Professor Richmond Sarpong.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门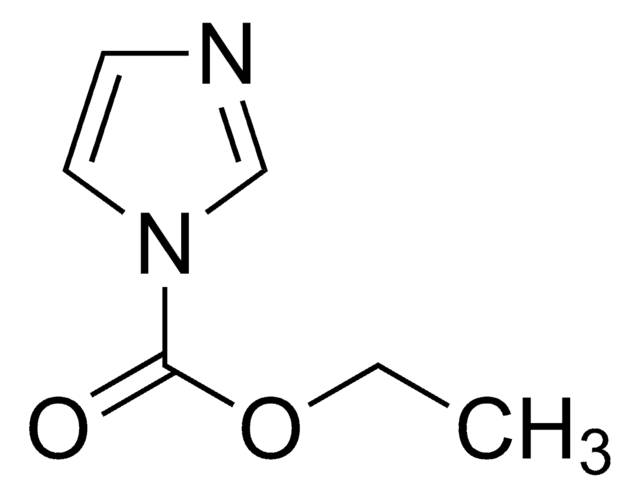



![4-叔丁基杯[4]芳烃四乙酸四乙酯 97%](/deepweb/assets/sigmaaldrich/product/structures/876/532/85287906-a532-4f7d-a312-a897fcbe6cea/640/85287906-a532-4f7d-a312-a897fcbe6cea.png)