推荐产品
化驗
97%
形狀
solid
mp
74-78 °C
SMILES 字串
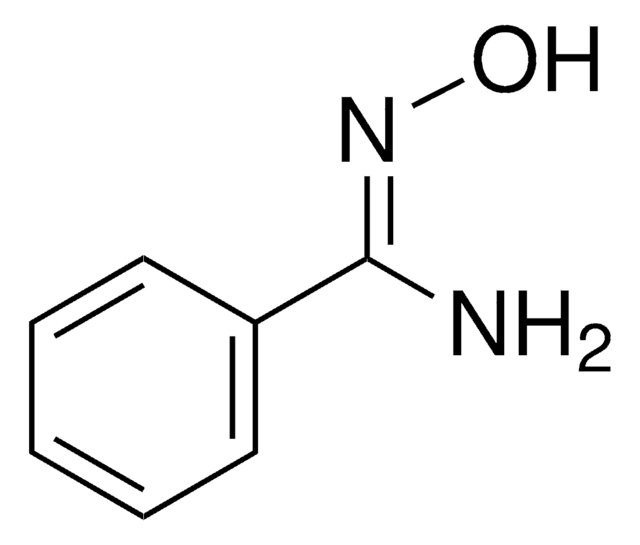
N\C(=N\O)c1ccccc1
InChI
1S/C7H8N2O/c8-7(9-10)6-4-2-1-3-5-6/h1-5,10H,(H2,8,9)
InChI 密鑰
MXOQNVMDKHLYCZ-UHFFFAOYSA-N
訊號詞
Danger
危險分類
Acute Tox. 3 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
A K Fröhlich et al.
Xenobiotica; the fate of foreign compounds in biological systems, 35(1), 17-25 (2005-03-25)
N-Hydroxyamidines (amidoximes) can act as pro-drugs of amidines (e.g. ximelagatran, a novel direct thrombin inhibitor). This known pro-drug principle is based on the N-reduction of an oral bioavailable amidoxime to its active form. Previous study of the metabolism of the
B Clement et al.
The Journal of biological chemistry, 272(31), 19615-19620 (1997-08-01)
Drugs containing strong basic nitrogen functional groups can be N-oxygenated to genotoxic products. While the reduction of such products is of considerable toxicological significance, most in vitro studies have focused on oxygen-sensitive reductase systems. However, an oxygen-insensitive microsomal hydroxylamine reductase
A Jousserandot et al.
Biochemistry, 37(49), 17179-17191 (1998-12-23)
Oxidation by rat liver microsomes of 13 compounds involving a C=N(OH) function (including N-hydroxyguanidines, amidoximes, ketoximes, and aldoximes) was found to occur with the release of nitrogen oxides such as NO, NO2-, and NO3-. The greatest activities were observed with
B Clement et al.
Archiv der Pharmazie, 322(7), 431-435 (1989-07-01)
At pH 7.4 neither benzamidine (1) is ring-hydroxylated nor benzamidoxime (2) is N-hydroxylated, reduced or ring-hydroxylated by aerobic incubations with microsomal fractions (12000 g supernatant, microsomes) of rabbit liver homogenates and NADPH. Products of hydrolytic processes are also not detected.
T L Deegan et al.
Bioorganic & medicinal chemistry letters, 9(2), 209-212 (1999-02-18)
1,2,4-Oxadiazoles have been prepared in parallel using 1,1'-carbonyldiimidazole (CDI) as a reagent for both formation and cyclodehydration of O-acyl benzamidoximes. The use of CDI facilitates parallel purification of the oxadiazole products by simple liquid-liquid extraction and filtration.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门