推荐产品
等級
technical
化驗
≥90% (HPLC)
形狀
crystals
mp
191-192 °C (dec.)
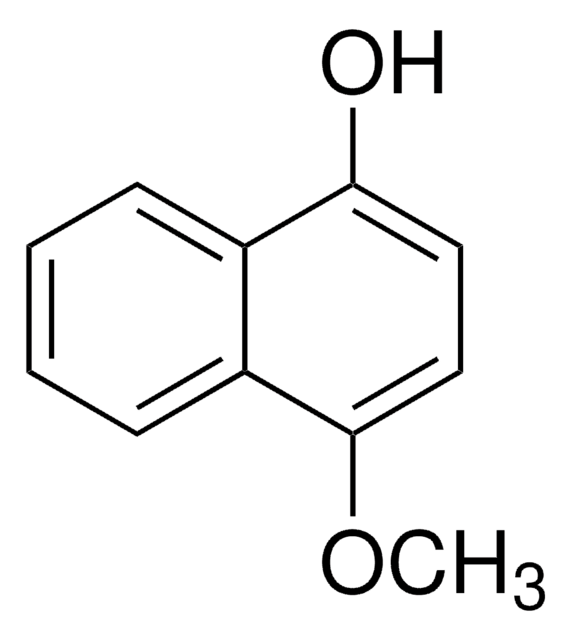
SMILES 字串
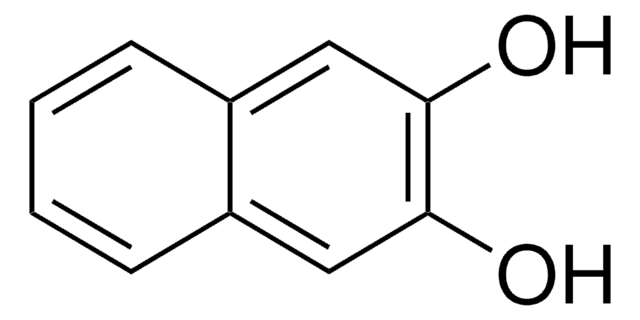
Oc1ccc(O)c2ccccc12
InChI
1S/C10H8O2/c11-9-5-6-10(12)8-4-2-1-3-7(8)9/h1-6,11-12H
InChI 密鑰
PCILLCXFKWDRMK-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
訊號詞
Danger
危險分類
Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Aurora Molinari et al.
Archiv der Pharmazie, 342(10), 591-599 (2009-09-16)
Several new 6-(3-pyrazolylpropyl) derivatives of 1,4-naphthohydroquinone-1,4-diacetate (NHQ-DA) have been prepared by chemical modifications of the Diels-Alder adduct of alpha-myrcene and 1,4-benzoquinone. All these new compounds and precursors have been evaluated in vitro for their cytotoxicity against cultured human cancer cells
L S Tsuruda et al.
Archives of toxicology, 69(6), 362-367 (1995-01-01)
Naphthalene (NA) is metabolically activated to the reactive intermediates, naphthalene oxide (NO) and naphthoquinones. To investigate the role of circulating reactive metabolites in NA toxicity, the half-life of NO was examined. The in vitro half-life of NO in both whole
Kevin W Wellington et al.
Bioorganic & medicinal chemistry, 20(14), 4472-4481 (2012-06-12)
Nuclear monoamination of a 1,4-naphthohydroquinone with primary aromatic amines was catalysed by the commercial laccase, Novozym 51003, from Novozymes to afford aminonaphthoquinones. The synthesis was accomplished by reacting a mixture of the primary amine and 1,4-naphthohydroquinone in succinate-lactate buffer and
T Ishii et al.
Free radical biology & medicine, 8(1), 21-24 (1990-01-01)
The autoxidation of 1,4-naphthohydroquinone, in a phosphate, EDTA buffer at pH 7.4, exhibits an autocatalysis whose lag phase becomes more pronounced in the presence of either the Cu,Zn- or the Mn-containing superoxide dismutases. In contrast, the autoxidation of a second
María Teresa Molina et al.
The Journal of organic chemistry, 74(24), 9573-9575 (2009-11-27)
The NHC-catalyzed conjugate hydroacylation of 1,4-naphthoquinones allows for the synthesis of monoacylated 1,4-dihydroxynaphthalene derivatives. These targets, difficult to prepare selectively by standard protocols, represent important intermediates in the elaboration of highly substituted 1,4-naphthoquinone derivatives, which constitute relevant pharmaceutical scaffolds. High
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门