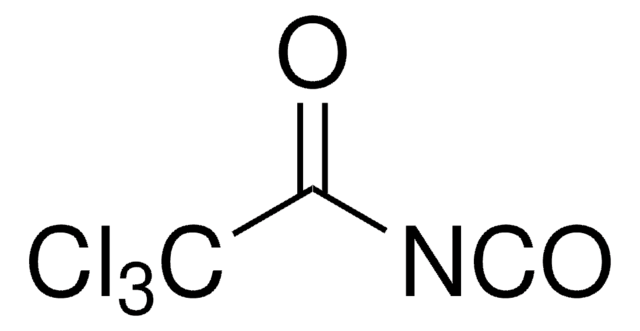
推荐产品
蒸汽壓力
5.57 psi ( 20 °C)
形狀
liquid
品質
Arxada quality
製造商/商標名
Arxada AG
濃度
99.0-100.3 % (w/w) (T)
雜質
≤1.00% chloropropylsulfonyl isocyanate
折射率
n20/D 1.447 (lit.)
bp
107 °C (lit.)
mp
−44 °C (lit.)
密度
1.626 g/mL at 25 °C (lit.)
SMILES 字串
ClS(=O)(=O)N=C=O
InChI
1S/CClNO3S/c2-7(5,6)3-1-4
InChI 密鑰
WRJWRGBVPUUDLA-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
- Pharmaceutical intermediates: A study explored the synthesis and carbonic anhydrase inhibitory properties of sulfamides derived from Chlorosulfonyl isocyanate, demonstrating its potential in the development of new therapeutic agents, particularly for treating conditions involving carbonic anhydrase enzymes (Aksu et al., 2013).
- Stereochemical synthesis applications: Chlorosulfonyl isocyanate was utilized for the stereoselective amination of chiral benzylic ethers in the total synthesis of (+)-sertraline, showcasing its critical role in the precise construction of complex pharmaceuticals (Lee et al., 2011).
- One-pot synthetic transformations: The compound′s use in one-pot conversion of trimethylsilyl ethers into urethanes was highlighted, further applied to synthesize novel neuromodulators like carisbamate. This illustrates Chlorosulfonyl isocyanate′s versatility in facilitating efficient and innovative synthetic routes in medicinal chemistry (Dong et al., 2008).
訊號詞
Danger
危險分類
Acute Tox. 4 Oral - Eye Dam. 1 - Resp. Sens. 1 - Skin Corr. 1B - Skin Sens. 1
安全危害
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
>230.0 °F
閃點(°C)
> 110 °C
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
J A Picard et al.
Journal of medicinal chemistry, 39(6), 1243-1252 (1996-03-15)
Several series of acyl-CoA:cholesterol O-acyltransferase inhibitors were prepared by the stepwise addition of nitrogen, oxygen, and sulfur nucleophiles to N-chlorosulfonyl isocyanate. The (aminosulfonyl)ureas 3-44 were the most potent inhibitors in vitro, with several compounds having IC50 values < 1 microM.
G Crassous et al.
Biomaterials, 5(3), 153-156 (1984-05-01)
In this paper we report the preparation of a new asymmetric semipermeable styrene-isoprene-styrene block-copolymer membrane. Its modification by addition of gaseous N-chlorosulphonylisocyanate alters neither its permselectivity nor its water permeability rate. This modified membrane possesses an antithrombic activity which depends
C P Sharma et al.
Biomaterials, medical devices, and artificial organs, 12(3-4), 215-233 (1984-01-01)
Natural rubber with C = C bonds had been modified by reaction with chlorosulfonyl isocyanate (CSI) and 70% of the products were obtained, which yielded polyelectrolyte on treatment with NaOH, having sulfamate and carboxylate groups. The polyelectrolyte showed anticoagulant activity.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门