推荐产品
光學活性
[α]20/D +14°, c = 1 in pentane
濃度
1 M in pentane
bp
35-36 °C
密度
0.735 g/mL at 25 °C
儲存溫度
−20°C
SMILES 字串
C[C@@H]1[C@H](C[C@H]2C[C@@H]1C2(C)C)B(CC=C)[C@H]3C[C@H]4C[C@@H]([C@@H]3C)C4(C)C
InChI
1S/C23H39B/c1-8-9-24(20-12-16-10-18(14(20)2)22(16,4)5)21-13-17-11-19(15(21)3)23(17,6)7/h8,14-21H,1,9-13H2,2-7H3/t14-,15-,16+,17+,18-,19-,20-,21-/m0/s1
InChI 密鑰
ZIXZBDJFGUIKJS-AXSQLCHVSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
應用
其他說明
訊號詞
Danger
危險分類
Aquatic Chronic 2 - Asp. Tox. 1 - Eye Irrit. 2 - Flam. Liq. 1 - Skin Irrit. 2 - STOT SE 3
標靶器官
Central nervous system, Respiratory system
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
-56.2 °F
閃點(°C)
-49 °C
個人防護裝備
Eyeshields, Faceshields, Gloves
相关内容
Asymmetric allylboration of aldehydes is an extremely important method for preparation of homoallylic alcohols, as evidenced in numerous complex natural product syntheses.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门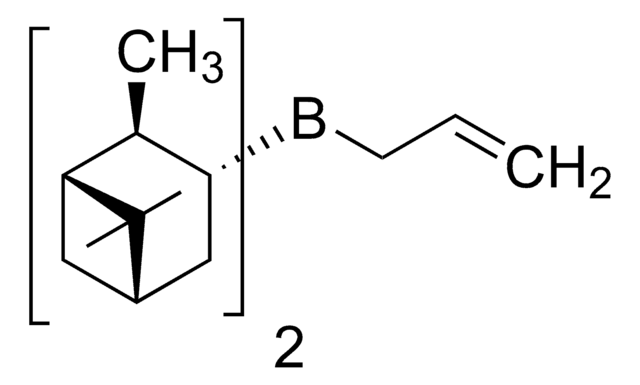




![9-硼双环[3.3.1]壬烷 溶液 0.5 M in THF](/deepweb/assets/sigmaaldrich/product/structures/180/891/8b64e597-269d-4780-98b6-40889dfd06b9/640/8b64e597-269d-4780-98b6-40889dfd06b9.png)





