所有图片(1)
About This Item
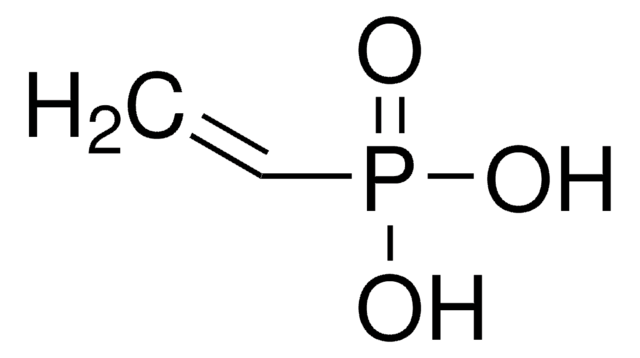
线性分子式:
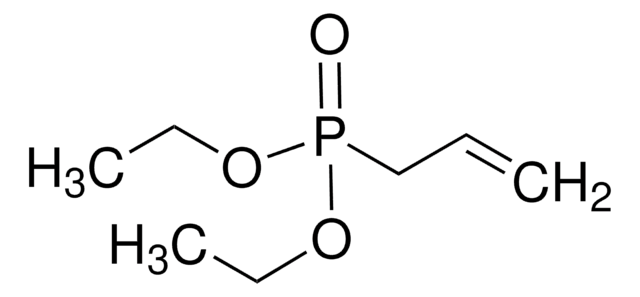
(C2H5O)2POCH2C(=CH2)CH3
CAS号:
分子量:
192.19
MDL號碼:
分類程式碼代碼:
12352108
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
97%
反應適用性
reaction type: C-C Bond Formation
折射率
n20/D 1.4380 (lit.)
bp
62 °C/0.1 mmHg (lit.)
密度
1.013 g/mL at 25 °C (lit.)
官能基
phosphonate
SMILES 字串
CCOP(=O)(CC(C)=C)OCC
InChI
1S/C8H17O3P/c1-5-10-12(9,11-6-2)7-8(3)4/h3,5-7H2,1-2,4H3
InChI 密鑰
QOZGSMHGXZMADD-UHFFFAOYSA-N
一般說明
Diethyl (2-methylallyl)phosphonate is an organophosphorous compound. It participates in the synthesis of α-aminophosphonate derivatives and azaphosphones. The analgesic/antiinflammatory properties of these derivatives were evaluated.
應用
Diethyl (2-methylallyl)phosphonate can be used as a reagent in the Horner-Wadsworth-Emmons reaction to form conjugated carbon–carbon double bonds.
It can also be used as a reactant for:
It can also be used as a reactant for:
- Enantioselective total synthesis of 10-isocyano-4-cadinene as antifouling agent.
- Regiospecific preparation of 4-oxo-2-alkenylphosphonates (OAP) via silylation followed by Friedel-Crafts acylation and isomerization. OAP can serve as building blocks for the construction of polyethylenic chains.
- The synthesis of azaphosphone as a potent analgesic/anti-inflammatory agents.
Reactant for:
- Enantioselective synthesis of 10-isocyano-4-cadinene and its stereoisomers with antifouling activity
- Preparation of 4-Oxo-2-alkenylphosphonates via silylation followed by regiospecific Friedel-Crafts acylation and isomerization
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
>230.0 °F - closed cup
閃點(°C)
> 110 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
Carbodiimides-key mediators in the synthesis of novel cytotoxic and analgesic/antiinflammatory motifs based on a-amino-, enaminophosphonates, and azaphosphones.
Abdou WM, et al.
Royal Society of Chemistry Advances, 3(5), 1528-1540 (2013)
I N Smirnova et al.
The journal of physical chemistry. B, 114(50), 16936-16947 (2010-12-01)
The high brilliance of the AILES beamline at the SOLEIL synchrotron facility has been exploited for the study of the gas-phase vibrational spectra of weakly volatile organophosphorous compounds. The propagation of the synchrotron radiation in long path length gas cells
Keisuke Nishikawa et al.
Organic letters, 12(5), 904-907 (2010-02-06)
The first enantioselective total synthesis of 10-isocyano-4-cadinene, a marine sesquiterpene isolated from nudibranchs of the family Phyllidiidae, was achieved. The cadinene is expected to be a novel nontoxic antifouling agent. In the synthesis, an intermolecular Diels-Alder reaction and a SmI(2)-induced
Lee et al.
The Journal of organic chemistry, 65(13), 4175-4178 (2000-06-24)
Treatment of allylic and vinylic phosphonates with excess LiHMDS, followed by addition of chlorotrimethylsilane, afforded alpha- and gamma- silylated allylic phosphonate mixtures. Without separation, these mixtures underwent the Friedel-Crafts reaction and base-promoted isomerization to give 4-oxo-2-alkenylphosphonates, which can serve as
(-)-Sparteine-mediated stereoselective intramolecular conjugate addition reactions of dienes and enynes
Oestreich M and Hoppe D
Tetrahedron Letters, 40(10), 1881-1884 (1999)
商品
傅-克酰基化反应是一种芳烃与酰氯或酸酐使用强路易斯酸催化剂的反应。该反应通过亲电芳族取代进行,形成单酰化产物。
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门