推荐产品
化驗
98%
形狀
solid
mp
149-152 °C (lit.)
SMILES 字串
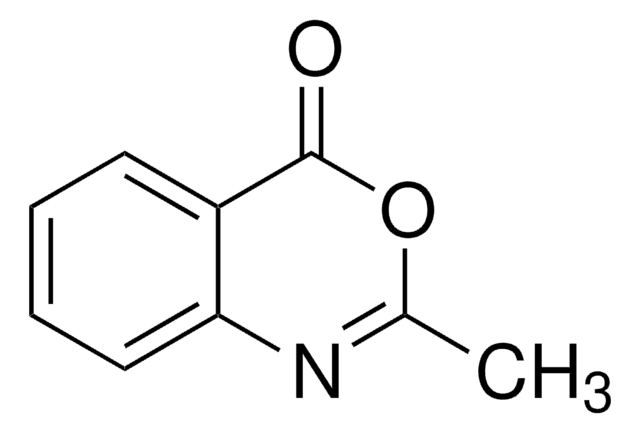
CC1=Nc2ccccc2C(=O)N1N
InChI
1S/C9H9N3O/c1-6-11-8-5-3-2-4-7(8)9(13)12(6)10/h2-5H,10H2,1H3
InChI 密鑰
IQOUPYQSZBDNAW-UHFFFAOYSA-N
一般說明
3-Amino-2-methyl-4(3H)quinazolinone is a quinazoline derivative. 2-Methyl-3,1-benzoxazin-4-one undergoes condensation reaction with hydrazine hydrate to yield 3-amino-2-methyl-4(3H)quinazolinone. It undergoes condensation with various substituted aldehydes to afford Schiff′s bases.
應用
3-Amino-2-methyl-4(3H)quinazolinone may be used to synthesize:
- 2-methyl-3-[3′-aminophthalimido]-4(3H)-quinazolinone
- 2-alkyl-3-(methylamino)-4(3H)-quinazolinone
- 3-amino-2-chloromethyl-4(3H)-quinazolinone
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Disperse dyes based on 2-methyl-3-[3'-amino-phthalimido]-4 (3H)-quinazilinone.
Patel VH, et al.
J. Serb. Chem. Soc., 67(11), 719-726 (2002)
Dipolar Cycloaddition Reactions with Quinazolinones: A New Route for the Synthesis of Several Annelated Pyrrolo-and Pyridazinoquinazoline Derivatives.
Ghabrial SS and Gaber HM.
Molecules (Basel), 8(5), 401-410 (2003)
Lithiation of 2-Alkyl-3-amino-and 2-Alkyl-3-(methylamino)-4 (3 H)-quinazolinones1.
Smith K, et al.
The Journal of Organic Chemistry, 61(2), 656-661 (1996)
Kollur Shiva Prasad et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 81(1), 276-282 (2011-07-12)
Four Schiff base ligands and their corresponding organotin(IV) complexes have been synthesized and characterized by elemental analyses, IR, (1)H NMR, MS and thermal studies. The Schiff bases are obtained by the condensation of 3-amino-2-methyl-4(3H)-quinazolinone with different substituted aldehydes. The elemental
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门