所有图片(2)
About This Item
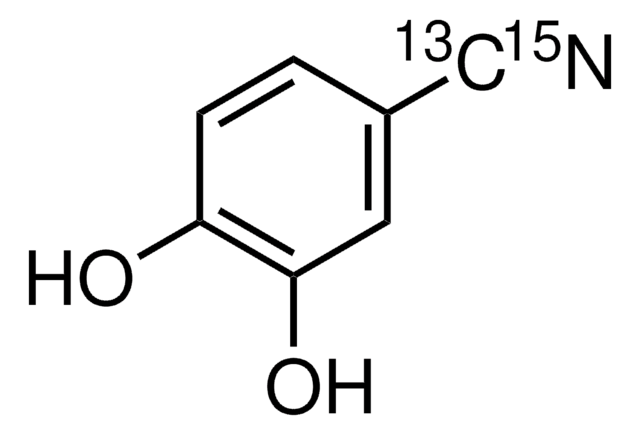
线性分子式:
(OH)2C6H3CN
CAS号:
分子量:
135.12
EC號碼:
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
97%
mp
155-159 °C (lit.)
SMILES 字串
Oc1ccc(cc1O)C#N
InChI
1S/C7H5NO2/c8-4-5-1-2-6(9)7(10)3-5/h1-3,9-10H
InChI 密鑰
NUWHYWYSMAPBHK-UHFFFAOYSA-N
一般說明
3,4-二羟基苄腈可由4-羟基-3-甲氧基苄腈制备。 它也可以通过3,4-二甲氧基苄腈、二异丙基氨基锂(LDA)和1,3-二甲基-2-咪唑烷酮(DMEU)的反应来合成。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
The synthetic technology of 3, 4-dihydroxybenzonitrile
WEI HW, et al.
Fine and Specialty Chemicals / Jing Xi Yu Zhuan Yong Hua Xue Pin, 9, 012-012 (2011)
Sodium Bis (trimethylsilyl) amide and Lithium Diisopropylamide in Deprotection of Alkyl Aryl Ethers: a-Effect of Silicon
Hwu JR, et al.
The Journal of Organic Chemistry, 62.12 , 4097-4104 (1997)
M J Nelson et al.
Biochemistry, 34(46), 15219-15229 (1995-11-21)
Ferric soybean lipoxygenase forms stable complexes with 4-substituted catechols. The structure of the complex between the enzyme and 3,4-dihydroxybenzonitrile has been studied by resonance Raman, electron paramagnetic resonance, visible, and X-ray spectroscopies. It is a bidentate iron-catecholate complex with at
M M Wick et al.
Journal of pharmaceutical sciences, 76(7), 513-515 (1987-07-01)
This report describes a structure-activity analysis of isomers of three classes of dihydroxybenzene derivatives, including dihydroxybenzaldoxime, dihydroxybenzaldehyde, and dihydroxybenzonitrile. These derivatives were examined for their effect on ribonucleotide reductase activity, macromolecular synthesis, cell growth, and in vivo antitumor activity against
The Reactivity and Reaction Pathway of Fenton Reactions Driven by Substituted 1,2-Dihydroxybenzenes.
Pablo Salgado et al.
Environmental science & technology, 51(7), 3687-3693 (2017-03-09)
Fenton systems are interesting alternatives to advanced oxidation processes (AOPs) applied in soil or water remediation. 1,2-Dihydroxybenzenes (1,2-DHBs) are able to amplify the reactivity of Fenton systems and have been extensively studied in biological systems and for AOP applications. To
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门