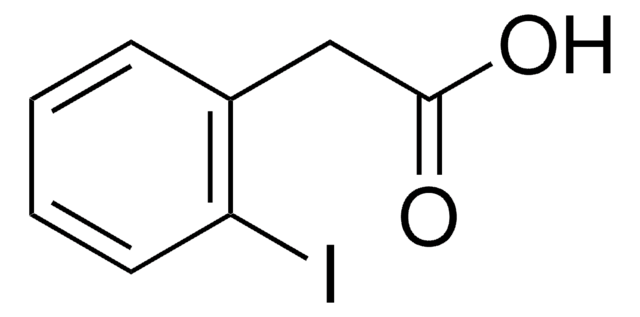
推荐产品
化驗
97%
折射率
n20/D 1.618 (lit.)
bp
113-120 °C/0.5 mmHg (lit.)
密度
1.75 g/mL at 25 °C (lit.)
SMILES 字串
Ic1ccccc1CC#N
InChI
1S/C8H6IN/c9-8-4-2-1-3-7(8)5-6-10/h1-4H,5H2
InChI 密鑰
FPSGTRJUQLYLHE-UHFFFAOYSA-N
一般說明
2-Iodophenylacetonitrile is a 2-aryl substituted nitrile. It reacts with lactams to form ring-fused isoquinolinones via palladium-catalyzed carboxamidation in tandem with aldol condensation.
應用
2-Iodophenylacetonitrile may be used in the preparation of:
It may also be used in the preparation of the following nitriles:
- 2?-aminobiphen-2-ylacetonitrile
- ethyl (2-iodophenyl)iminoacetate hydrochloride
- 3,4-disubstituted 2-naphthalenamines
It may also be used in the preparation of the following nitriles:
- 2-(2-iodophenyl)-2-methylpropanenitrile
- 1-(2-iodophenyl)cyclopentanecarbonitrile
- 5-bromo-2-(2-iodophenyl)pentanenitrile
- 2-(2-iodophenyl)-2-propylpentanenitrile
- 1-(2-iodophenyl)cyclohexanecarbonitrile
- 1-(2-Iodophenyl)cyclopropanecarbonitrile
訊號詞
Warning
危險分類
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
230.0 °F - closed cup
閃點(°C)
110 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Studies in Acyl C? H Activation via Aryl and Alkyl to Acyl ?Through Space? Migration of Palladium.
Kesharwani T, et al.
Organic Letters, 11(12), 2591-2593 (2009)
Palladium-catalyzed borylation of ortho-substituted phenyl halides and application to the one-pot synthesis of 2,2'-disubstituted biphenyls.
Baudoin O, et al.
The Journal of Organic Chemistry, 65(26), 9268-9271 (2000)
Hirokazu Tsukamoto et al.
The Journal of organic chemistry, 81(5), 1733-1745 (2015-11-26)
1,2-Bis(diphenylphosphino)ethane (dppe)-ligated palladium(II) complexes catalyze the annulation of internal alkynes with 2-(cyanomethyl)phenylboronates to provide 3,4-disubstituted-2-naphthalenamines in good yields. The annulation reaction proceeds under mild and neutral conditions and requires methanol as an essential solvent. In addition to symmetrical alkynes, unsymmetrical
David Crich et al.
The Journal of organic chemistry, 71(9), 3452-3463 (2006-04-22)
The [1-cyano-2-(2-iodophenyl)]ethylidene group is introduced as an acetal-protecting group for carbohydrate thioglycoside donors. The group is easily introduced under mild conditions, over short reaction times, and in the presence of a wide variety of other protecting groups by the reaction
Palladium-catalyzed carboxamidation reaction and aldol condensation reaction cascade: A facile approach to ring-fused isoquinolinones.
Chouhan G and Alper H.
Organic Letters, 10(21), 4987-4990 (2008)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门