所有图片(1)
About This Item
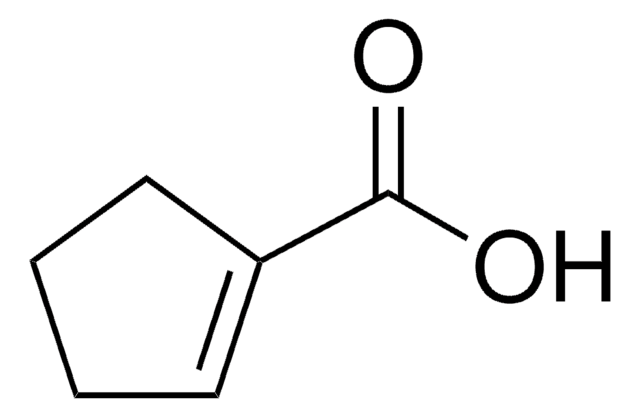
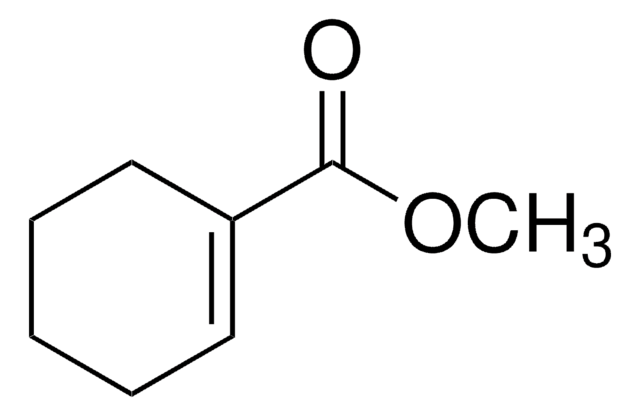
线性分子式:
C5H7CO2CH3
CAS号:
分子量:
126.15
Beilstein:
2040162
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
97%
形狀
liquid
折射率
n20/D 1.4660 (lit.)
bp
76-78 °C/9 mmHg (lit.)
密度
1.031 g/mL at 20 °C (lit.)
SMILES 字串
COC(=O)C1=CCCC1
InChI
1S/C7H10O2/c1-9-7(8)6-4-2-3-5-6/h4H,2-3,5H2,1H3
InChI 密鑰
VTYCAXIAUKEGBQ-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
Methyl 1-cyclopentene-1-carboxylate, a cyclic alkene, is a cyclopentene derivative. It participates in the synthesis of azulene derivatives by initially forming the corresponding cyclopropanol followed by oxy-Cope rearrangement.
應用
Methyl 1-cyclopentene-1-carboxylate may be used as starting material in the synthesis of pinnaic acid and halichlorine. It undergoes asymmetric oxidative Heck reaction with aryl boronic acids to form coupling products in the presence of chiral NHC (N-heterocyclic carbine)-palladium (II) complex.
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
143.6 °F - closed cup
閃點(°C)
62 °C - closed cup
個人防護裝備
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
THE ORGANIC CHEMISTRY NOTEBOOK SERIES, A DIDACTICAL APPROACH,?(I) A THEORETICAL MECHANISTIC APPROACH TO DIASTEROSELECTIVE SYNTHESIS OF CIS-1, 2-DIALKENYLCYCLOPROPANOLS AND SUBSEQUENT OXY-COPE REARRANGEMENT BY JIN KUN CHA ET AL.
Bravo J.
Revista Boliviana de Quimica, 22(1), 1-10 (2005)
Rodrigo B Andrade et al.
Organic letters, 7(25), 5733-5735 (2005-12-03)
[chemical reaction: see text]. Concise formal syntheses of marine alkaloids (+/-)-pinnaic acid (1) and (+/-)-halichlorine (2) have been accomplished from a common intermediate. The syntheses illustrate the utility of selective olefin cross metathesis methodologies for the elaboration of advanced synthetic
Kyung Soo Yoo et al.
The Journal of organic chemistry, 75(1), 95-101 (2009-12-04)
Chiral dimeric tridentate NHC-amidate-alkoxide palladium(II) complexes, 3a and 3b, effected oxidative boron Heck-type reactions of aryl boronic acids with both acyclic and cyclic alkenes at room temperature to afford the corresponding coupling products with high enantioselectivities. The high degree of
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门