推荐产品
等級
catalyst grade (for peroxide polymerization)
蒸汽密度
>1 (vs air)
化驗
≥98.5% (GC)
形狀
liquid
expl. lim.
7 %
折射率
n20/D 1.546 (lit.)
n20/D 1.547
bp
211 °C (lit.)
90-92 °C/10 mmHg (lit.)
密度
0.936 g/mL at 20 °C
0.937 g/mL at 25 °C (lit.)
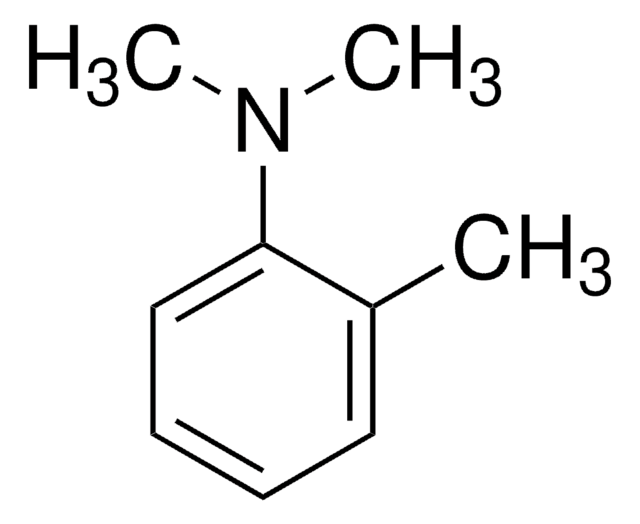
SMILES 字串
CN(C)c1ccc(C)cc1
InChI
1S/C9H13N/c1-8-4-6-9(7-5-8)10(2)3/h4-7H,1-3H3
InChI 密鑰
GYVGXEWAOAAJEU-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
4,N,N-Trimethylaniline is a N-methyl-N-alkylaniline. Its reaction with vinyl ether catalyzed by CuCl2 has been reported to afford tetrahydroquinolines. Its radical cation undergoes reaction with the anthracene radical anion and generation of electrogenerated chemiluminescence (ECL) has been observed.
應用
- Charge-transfer complexes for redox polymerization: 4,N,N-Trimethylaniline used for on-demand amine/peroxide redox polymerization. This research offers a new perspective on the use of 4,N,N-Trimethylaniline in creating controlled polymer structures, which is crucial for various industrial and pharmaceutical applications (Garra et al., 2018).
注意
储存中可能变成黄绿色
訊號詞
Danger
危險分類
Acute Tox. 2 Inhalation - Acute Tox. 3 Dermal - Acute Tox. 3 Oral - Aquatic Chronic 3 - Carc. 1B - Repr. 2 - Skin Sens. 1 - STOT RE 2 Oral
標靶器官
Reproductive organs
儲存類別代碼
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
168.8 °F - closed cup
閃點(°C)
76 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Jacob B Ketter et al.
Journal of the American Chemical Society, 126(32), 10183-10189 (2004-08-12)
Electrogenerated chemiluminescence (ECL) arising from the reaction of radical ions has previously be shown to arise from a variety of states including excited singlets, triplets, excimers, and exciplexes. In this work we describe two systems that form emissive states in
Xianghua Yang et al.
Molecules (Basel, Switzerland), 11(12), 978-987 (2007-11-17)
Tetrahydroquinoline skeletons can be formed by a CuCl2-catalyzed one-pot reaction of N-methyl-N-alkylanilines and vinyl ethers in the presence of t-butyl-hydroperoxide.
Sachiko Kaihara et al.
European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 68(1), 67-73 (2007-09-25)
While many synthetic, hydrolytically degradable hydrogels have been developed for biomedical applications, there are only a few examples whose polymer backbone does not form acidic products upon degradation. In order to address this concern, we proposed to develop a hydrogel
Anuradha Prakki et al.
Dental materials : official publication of the Academy of Dental Materials, 25(1), 26-32 (2008-09-02)
The purpose of this study was to evaluate the effect of two additives, propionaldehyde/aldehyde or 2,3-butanedione/diketone, on mechanical properties of Bis-GMA-based composites containing TEGDMA, propoxylated Bis-GMA (CH(3)Bis-GMA) or propoxylated fluorinated Bis-GMA (CF(3)Bis-GMA). Three control composites, Bis-GMA/diluent monomer (25/75 mol%), and
Kyle Winter et al.
Biomaterials, 26(26), 5321-5329 (2005-04-09)
Previous investigations have found that visible-light (VL)-irradiated camphorquinone (CQ), in the presence of a tertiary amine (e.g., N,N-dimethyl-p-toluidine, DMT), generates reactive oxygen species and causes oxidative DNA damage in vitro. In this study, oxidative DNA damage produced by VL-irradiated CQ/DMT
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门