所有图片(1)
About This Item
经验公式(希尔记法):
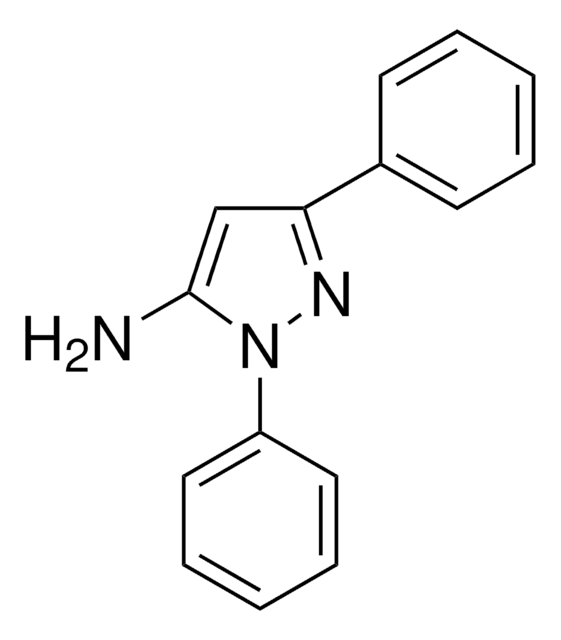
C9H9N3
CAS号:
分子量:
159.19
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
98%
mp
124-127 °C (lit.)
SMILES 字串
Nc1cc([nH]n1)-c2ccccc2
InChI
1S/C9H9N3/c10-9-6-8(11-12-9)7-4-2-1-3-5-7/h1-6H,(H3,10,11,12)
InChI 密鑰
PWSZRRFDVPMZGM-UHFFFAOYSA-N
一般說明
3-Amino-5-phenylpyrazole (3-phenyl-1H-pyrazol-5-amine), an amino pyrazole derivative, is an aza-heterocyclic amine. It has been reported to be synthesized by heating either 3-amino-4-bromo- or 3-amino-5-phenylisothiazole in the presence of anhydrous hydrazine. On reaction with ZnCl2 it affords chlorido-tris(3-amino-5-phenyl-1Hpyrazole-N2)zinc (II) chloride.
應用
3-Amino-5-phenylpyrazole ((3-phenyl-1H-pyrazol-5-amine) may be used in the synthesis of the following:
- Urea derivatives by reaction with azido(6-(benzofuran-2-yl)-2-methylpyridin-3-yl) methanone.
- 2-Mercaptoacetamide analogs by treating with thioglycolic acid.
- 3-(Substituentpyrimidayl)-5,6-benzocoumarins by treating with 3-(2′-formyl-1′-chlorovinyl)-5,6-benzocoumarin.
- Substituted 2,7-diphenylpyrazolo[1,5-a]pyrimidine-5-carboxylic esters by reacting with substituted β-diketo esters.
- N-ethoxycarbonylthiourea derivative by reacting with ethoxycarbonyl isothiocyanate.
- Heterobiaryl pyrazolo[3,4-b]pyridines by reacting with indole-3-carboxaldehyde.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Crystal structure of chlorido-tris (3-amino-5-phenyl-1H pyrazole-N2) zinc (II) chloride, [ZnCl (C9H9N3)3] Cl.
Jacimovic ZK, et al
Zeitschrift fur Kristallographie, 226(3), 397-399 (2011)
The conversion of isothiazoles into pyrazoles using hydrazine.
Ioannidou HA and Koutentis PA
Tetrahedron, 65(34), 7023-7037 (2009)
Scott T Moe et al.
Bioorganic & medicinal chemistry, 17(8), 3072-3079 (2009-03-31)
Botulinum neurotoxin elicits its paralytic activity through a zinc-dependant metalloprotease that cleaves proteins involved in neurotransmitter release. Currently, no drugs are available to reverse the effects of botulinum intoxication. Herein we report the design of a novel series of mercaptoacetamide
Recent advances in the chemistry of ethoxycarbonyl isothiocyanate and related compounds.
George B and Papadopoulos EP
Journal of Heterocyclic Chemistry, 20(5), 1127-1142 (1983)
Convenient synthesis of some new pyrazolo [5, 1-c] triazines, isoxazolo [3, 4-d] pyrimidine and pyridine derivatives containing benzofuran moiety.
Abdelhamid AO, et al
European Journal of Chemistry, 3(2), 129-137 (2012)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门