所有图片(1)
About This Item
经验公式(希尔记法):
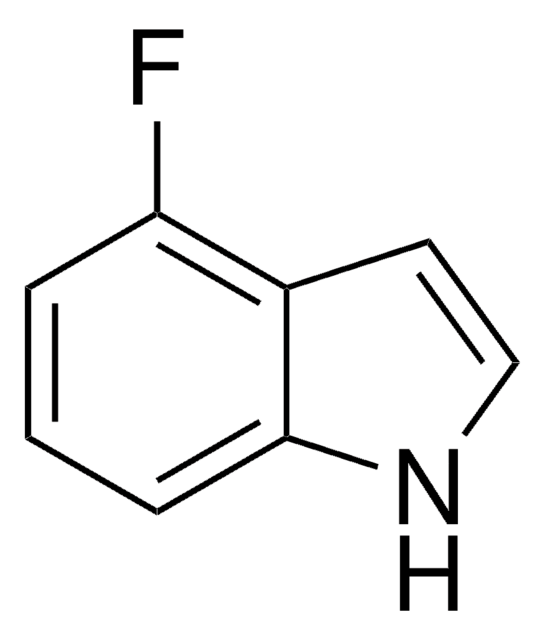
C8H6FN
CAS号:
分子量:
135.14
Beilstein:
112192
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
98%
mp
72-76 °C (lit.)
SMILES 字串
Fc1ccc2cc[nH]c2c1
InChI
1S/C8H6FN/c9-7-2-1-6-3-4-10-8(6)5-7/h1-5,10H
InChI 密鑰
YYFFEPUCAKVRJX-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
6-Fluoroindole is a halogen substituted indole. Experimental ionization potential of 6-fluoroindole has been evaluated. Preparation of 6-fluoroindole via nitration of indoline has been reported.
應用
6-Fluoroindole may be used as reactant in the preparation of:
- tryptophan dioxygenase inhibitors pyridyl-ethenyl-indoles as potential anticancer immunomodulators
- antibacterial agents
- antifungal agents
- Sodium-Dependent Glucose Co-transporter 2 (SGLT2) Inhibitors for the management of hyperglycemia in diabetes
- potent selective serotonin reuptake inhibitors
- inhibitors of HIV-1 attachment
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Na, Y. M.
Bull. Korean Chem. Soc., 31, 3467-3467 (2010)
S Jimmy Budiardjo et al.
ACS synthetic biology, 5(12), 1475-1484 (2016-07-09)
Chemical biology has long sought to build protein switches for use in molecular diagnostics, imaging, and synthetic biology. The overarching challenge for any type of engineered protein switch is the ability to respond in a selective and predictable manner that
David F Cummings et al.
Bioorganic & medicinal chemistry, 18(13), 4783-4792 (2010-06-24)
Efforts to develop ligands that distinguish between clinically relevant 5-HT2A and 5-HT2C serotonin receptor subtypes have been challenging, because their sequences have high homology. Previous studies reported that a novel aplysinopsin belonging to a chemical class of natural products isolated
G S Sheppard et al.
Journal of medicinal chemistry, 37(13), 2011-2032 (1994-06-24)
(2RS,4R)-3-(2-(3-Pyridinyl)thiazolidin-4-oyl)indoles represent a new class of potent, orally active antagonists of platelet activating factor (PAF). The compounds were prepared by acylation of the magnesium or zinc salts of substituted indoles with (2RS,4R)-2-(3-pyridinyl)-3-(tert-butoxycarbonyl)thiazolidin-4-oyl chloride. The 3-acylindole moiety functions as a hydrolytically
Journal of Medicinal Chemistry, 36, 2242-2242 (1993)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门