推荐产品
化驗
99%
光學活性
[α]20/D +145°, c = 2 in acetone
光學純度
ee: 99% (HPLC)
mp
73-75 °C (lit.)
SMILES 字串
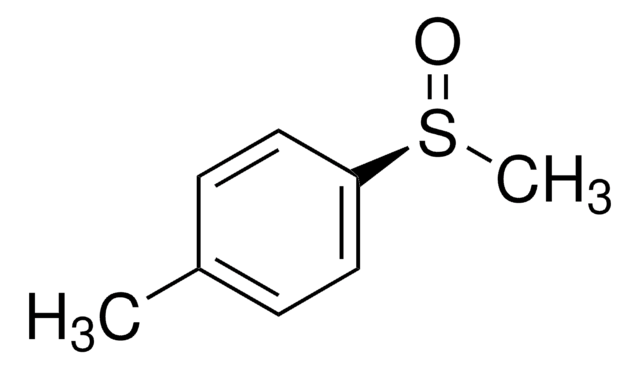
Cc1ccc(cc1)S(C)=O
InChI
1S/C8H10OS/c1-7-3-5-8(6-4-7)10(2)9/h3-6H,1-2H3/t10-/m1/s1
InChI 密鑰
FEVALTJSQBFLEU-SNVBAGLBSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
(R)-(+)-Methyl p-tolyl sulfoxide may be used to prepare (R)-(+)-methyl 3,5-dimethoxy-6-[8-oxo-9-(p-tolylsulfinyl) nonyl] benzoate, an intermediate for (R)-lasiodiplodin synthesis. Its anions undergo addition reaction with nitrones to form optically active a-substituted N-hydroxylamines. (R)-(+)-Methyl p-tolyl sulfoxide also reacts with O-mesitylsulfonylhydroxylamine (MSH) to form (-)-(R)-S-methyl-S-p-tolylsulfoximine.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
Asymmetric synthesis of orsellinic acid type macrolides: The example of lasiodiplodin.
Solladie G, et al.
Tetrahedron Asymmetry, 1(3), 187-198 (1990)
Chemistry of sulfoxides and related compounds. XLIX. Synthesis of optically active sulfoximines from optically active sulfoxides.
Johnson CR, et al.
The Journal of Organic Chemistry, 39(16), 2458-2459 (1974)
The reaction of nitrones with (R)-(+)-methyl p-tolyl sulfoxide anion; asymmetric synthesis of optically active secondary amines.
Murahashi S-I, et al.
Tetrahedron Letters, 34(16), 2645-2648 (1993)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门