所有图片(1)
About This Item
经验公式(希尔记法):
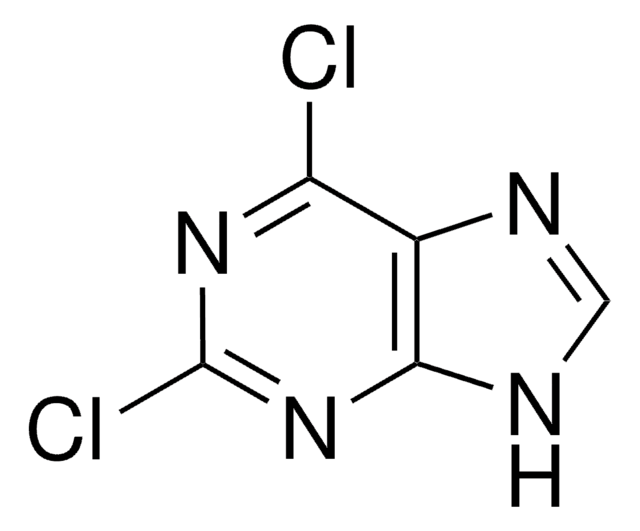
C5H4ClN5
CAS号:
分子量:
169.57
Beilstein:
9626
EC號碼:
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
品質等級
化驗
97%
mp
>300 °C (lit.)
SMILES 字串
Nc1nc(Cl)c2nc[nH]c2n1
InChI
1S/C5H4ClN5/c6-3-2-4(9-1-8-2)11-5(7)10-3/h1H,(H3,7,8,9,10,11)
InChI 密鑰
RYYIULNRIVUMTQ-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
2-氨基-6-氯嘌呤是一种 6-取代嘌呤。用振动光谱和量子化学方法研究了 2-氨基-6-氯嘌呤的互变异构体。
應用
2-氨基-6-氯嘌呤可用于:
- 酶促合成 2′-脱氧鸟苷
- 9-烷基嘌呤的合成
- 嘌呤和嘧啶碱 ( R )-和 ( S )- N -(2-磷酸甲氧基丙基)衍生物的合成
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
Synthesis of enantiomeric N-(2-phosphonomethoxypropyl) derivatives of purine and pyrimidine bases. II. The synthon approach.
Holy A, et al.
Collection of Czechoslovak Chemical Communications, 60(8), 1309-1409 (1995)
K Kim et al.
Combinatorial chemistry & high throughput screening, 3(2), 125-129 (2000-05-02)
A new application of solid-supported reagents was developed to separate the alkylated N7/N9 regioisomers derived from commercially available 2-amino-6-chloropurine. Simple filtration through an alumina/H+ pad or scavenging by AG/Dowex-50W-X8 resin provides diverse N9 regioisomers selectively in moderate yields with high
Jan Novák et al.
Organic letters, 5(5), 637-639 (2003-02-28)
Protecting the hydroxyl group in both 2-bromo-2-phenylethanol and 2-bromo-1-phenylethanol enhanced the alkylation of 2-amino-6-chloropurine to give corresponding 7- and 9-alkylated products. Subsequent hydrolysis and deprotection led to 7- and 9-hydroxy(phenyl)ethylguanines. 7-Hydroxy(phenyl)ethylguanines are major guanine adducts formed by interaction of styrene
L L Bennett et al.
Biochemical pharmacology, 33(2), 261-271 (1984-01-15)
2-Amino-6-chloro-1-deazapurine is of interest as a purine analog with demonstrated in vivo activity against mouse leukemia L1210. That the active form of this agent is a nucleotide and that the nucleotide is formed by the action of hypoxanthine (guanine) phosphoribosyltransferase
V Balachandran et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 96, 340-351 (2012-06-19)
Two purine tautomers of 2-amino-6-chloropurine (ACP), in labeled as N(9)H(10) and N(7)H(10), were investigated by vibrational spectroscopy and quantum chemical method. The FT-IR and FT-Raman spectra of ACP have been recorded in the regions 4000-400 cm(-1) and 3500-100 cm(-1), respectively.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门