所有图片(1)
About This Item
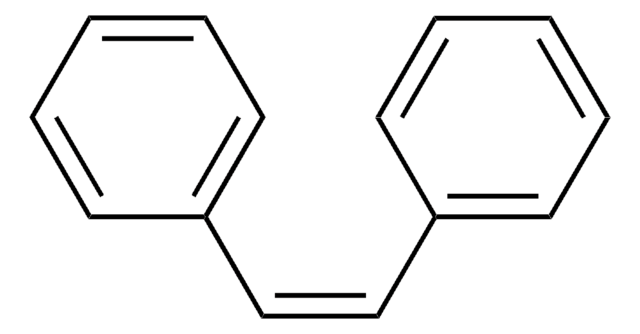
经验公式(希尔记法):
C14H12O
CAS号:
分子量:
196.24
Beilstein:
82737
MDL號碼:
分類程式碼代碼:
12162002
PubChem物質ID:
NACRES:
NA.23
推荐产品
化驗
97%
mp
38-40 °C (lit.)
儲存溫度
2-8°C
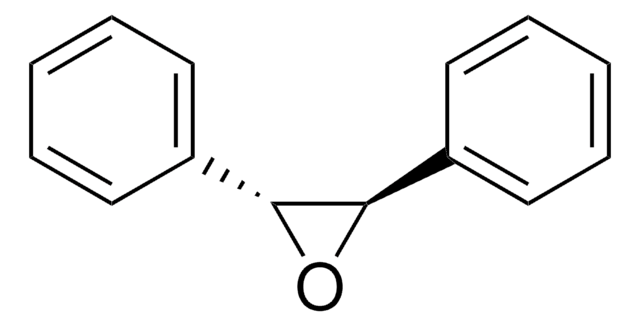
SMILES 字串
O1[C@H]([C@H]1c2ccccc2)c3ccccc3
InChI
1S/C14H12O/c1-3-7-11(8-4-1)13-14(15-13)12-9-5-2-6-10-12/h1-10,13-14H/t13-,14+
InChI 密鑰
ARCJQKUWGAZPFX-OKILXGFUSA-N
正在寻找类似产品? 访问 产品对比指南
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
228.2 °F - closed cup
閃點(°C)
109 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
Ylva Ivarsson et al.
Biochimica et biophysica acta, 1770(9), 1374-1381 (2007-08-11)
Based on the crystal structure of human glutathione transferase M1-1, cysteine residues were introduced in the substrate-binding site of a Cys-free mutant of the enzyme, which were subsequently alkylated with 1-iodoalkanes. By different combinations of site-specific mutations and chemical modifications
Richard Lonsdale et al.
Biochemistry, 51(8), 1774-1786 (2012-01-28)
Soluble epoxide hydrolase (sEH) is an enzyme involved in drug metabolism that catalyzes the hydrolysis of epoxides to form their corresponding diols. sEH has a broad substrate range and shows high regio- and enantioselectivity for nucleophilic ring opening by Asp333.
Paloma Vidal et al.
The Journal of organic chemistry, 72(9), 3166-3170 (2007-03-10)
This study presents a simple method for measuring long-range heteronuclear coupling constants between protons and proton-bearing carbons. The approach involves recording two conventional 1D-TOCSY experiments in which the offset of the selective proton pulse is set on the low- and
Kouhei Shimomura et al.
Nature chemistry, 6(5), 429-434 (2014-04-24)
In the chromatographic separation of enantiomers the order of elution is determined by the strength of diasteromeric interactions between the components of the mixture and a chiral stationary phase. For analytical purposes, it is ideal to have the minor component
Vito Capriati et al.
Organic letters, 7(22), 4895-4898 (2005-10-21)
[reaction: see text] A stereoselective/stereospecific synthesis of polysubstituted tetrahydronaphthols based on the Michael addition of ortho-lithiated stilbene oxides to alpha,beta-unsaturated Fischer carbene complexes followed by an unusual cyclization of the corresponding intermediate in a 6-endo-tet mode is described.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门