所有图片(1)
About This Item
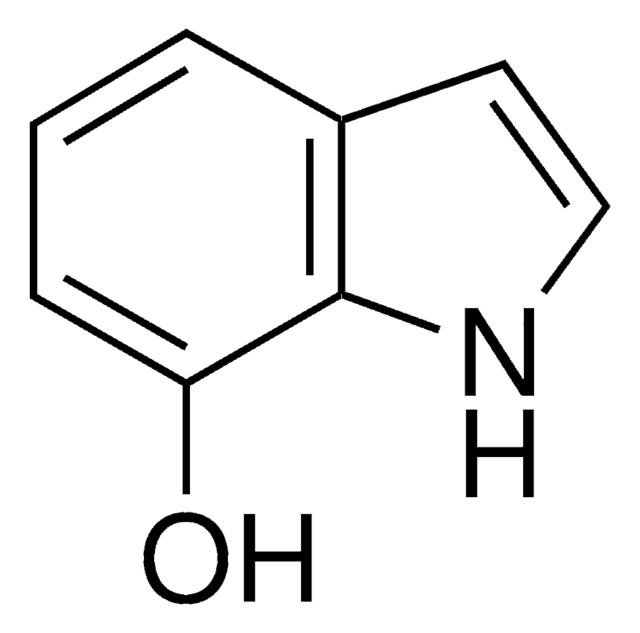
经验公式(希尔记法):
C10H11NO2
CAS号:
分子量:
177.20
Beilstein:
5683
EC號碼:
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
99%
形狀
solid
mp
154-157 °C (lit.)
SMILES 字串
COc1cc2cc[nH]c2cc1OC
InChI
1S/C10H11NO2/c1-12-9-5-7-3-4-11-8(7)6-10(9)13-2/h3-6,11H,1-2H3
InChI 密鑰
QODBZRNBPUPLEZ-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
5,6-Dimethoxyindole was used in the synthesis of N-benzyl-N-cyclopropyl-5,6-dimethoxyindole-3-glyoxalamide.
- Reactant in synthesis of indolylhydroxyoxindoles via enantioselective Friedel-Crafts reaction
- Reactant in synthesis of benzyl trimethoxyindoles
- Reactant for synthesis of benzoylpiperazinyl-indolyl ethane dione derivatives as HIV-1 inhibitors
- Reactant for synthesis of 1-aroylindole 3-aroylindoles combretastatin A-4 analogs as antitumor agents and tubulin polymerization inhibitors
- Reactant for preparation of tryptophanol derivatives via the Grignard reaction
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
F Chimenti et al.
Il Farmaco; edizione scientifica, 35(9), 785-790 (1980-09-01)
The synthesis of two new N-cyclopropyltryptamines is described. By treating 5,6-dimethoxyindole with oxalyl chloride and N-benzylcyclopropylamine, N-benzyl-N-cyclopropyl-5,6-dimethoxyindole-3-glyoxalamide is obtained. The reduction of this compound by LiAlH4, gives N-benzyl-N-cyclopropyl-5,6-dimethoxytryptamine, which is hydrogenated to N-cyclopropyl-5,6-dimethoxytryptamine. Similarly N-cyclopropyl-6,7-dimethoxytryptamine is prepared. Preliminary results indicate
J Hirschinger et al.
Solid state nuclear magnetic resonance, 3(3), 121-135 (1994-06-01)
The inversion-recovery cross-polarization (IRCP) magic-angle spinning experiment has been applied to study the 13C-1H cross-polarization dynamics of protonated aromatic carbons in ferrocene, 5,6-dimethoxyindole (DMI) and some indole derivatives. Using the 13C-detected proton spin diffusion (SD) experiment recently developed by Zhang
W B Emary et al.
Rapid communications in mass spectrometry : RCM, 3(12), 413-416 (1989-12-01)
It is not possible to distinguish isomers of biologically important dimethoxyindoles using electron-ionization mass spectra, but they may be distinguished by collisionally activated dissociation. In particular, energy-resolved mass spectrometry yields the best data for distinguishing between these isomers.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门